Chiral Separation and Determination of Etoxazole Enantiomers in Vegetables by Normal-Phase and Reverse-Phase High Performance Liquid Chromatography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ERMA200695 E and R Report
ERMA New Zealand Evaluation and Review Report Application for approval to import or manufacture ParaMite Selective Miticide for release Application Number: ERMA200695 Executive Summary Background information Sumitomo Chemical Australia Pty Ltd is seeking approval to import or manufacture ParaMite Selective Miticide for release. ParaMite Selective Miticide is a soluble concentrate containing 110 g/litre etoxazole and other ingredients. ParaMite Selective Miticide is intended for the control of Six-Spotted Mites in avocados. ParaMite Selective Miticide will be applied by ground-based methods only, at a maximum application rate of 1.05 L/ha (121.6 g ai/ha), 1 time per year. Etoxazole is a new active ingredient to New Zealand. Classification The Agency has classified ParaMite Selective Miticide based on the composition of ParaMite Selective Miticide and the effects of its components. Hazardous Property Assessment Target Organ Toxicity 6.9A Aquatic Ecotoxicity 9.1A Risk Assessment The Agency‘s assessment of the risks posed by ParaMite Selective Miticide to the environment and to human health, during the substance‘s lifecycle, is based on qualitative assessment and quantitative modelling using the GENEEC2 and German BBA models. The Agency considers that the risk assessments indicate that the risks to human health and the environment posed by ParaMite Selective Miticide are negligible with the proposed controls in place. The Agency has evaluated information supplied by the applicant about the benefits of ParaMite Selective Miticide and considers -

Certificate of Analysis
CERTIFICATE OF ANALYSIS IDENTIFICATION: Page 1 of 3 PRODUCT NAME: XJ-13 PRODUCT DESIGNATION: Proprietary Strain Terpene Blend Profile SKU #: GCS-XJ13 INTENDED FOR USE BY: December 2021 LOT #: GCT10060 CAS #: Mixture EC #: Mixture PARAMETER: SPECIFICATION: RESULTS: APPEARANCE: Clear, Light Yellow Liquid Clear, Light Yellow Liquid ODOR: Citrus, Pine & Pungent Citrus, Pine & Pungent ADDITIONAL PRODUCT INFORMATION: Storage Conditions: Stable when stored in its original container securely tightened and away from heat, open flames, sunlight, combustible materials and hot surfaces. Store in a cool, dry, and well-ventilated place. TRACE CONTAMINANT LEVELS: Test Type: Heavy Metals Contaminant Name Max. Allowed (ppm) Test Result Contaminant Name Max. Allowed (ppm) Test Result Arsenic 0.2 PASSED Cadmium 0.2 PASSED Lead 0.5 PASSED Mercury 0.1 PASSED Test Type: Pesticide Contaminant Name Max. Allowed (ppm) Test Result Contaminant Name Max. Allowed (ppm) Test Result Abamectin 0.07 PASSED Acephate 0.1 PASSED Acequinocyl 0.1 PASSED Acetamiprid 0.1 PASSED Aldicarb 0.4 PASSED Azoxystrobin 0.02 PASSED Bifenazate 0.02 PASSED Bifenthrin 0.1 PASSED Boscalid 0.1 PASSED Captan 0.7 PASSED Gold Coast Terpenes LLC // 1201 N Catalina Ave. PO Box 3435, Redondo Beach, CA 90277 goldcoastterpenes.com // [email protected] // 929-356-6086 CERTIFICATE OF ANALYSIS Test Type: Pesticide (Continued) Page 2 of 3 Contaminant Name Max. Allowed (ppm) Test Result Contaminant Name Max. Allowed (ppm) Test Result Carbaryl 0.2 PASSED Carbofuran 0.2 PASSED Chlorantraniliprole -

Determination of Etoxazole Residues in Fruits and Vegetables by SPE Clean
This article was downloaded by: [Enstinet], [Farag Malhat] On: 25 February 2013, At: 03:14 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Journal of Environmental Science and Health, Part B: Pesticides, Food Contaminants, and Agricultural Wastes Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/lesb20 Determination of etoxazole residues in fruits and vegetables by SPE clean-up and HPLC-DAD Farag Malhat a , Hany Badawy b , Dalia Barakat b & Ayman Saber a a Pesticide Residues and Environmental Pollution Department, Agriculture Research Center, Dokki, Giza, Egypt b Pesticides & Economic Insect Department, Faculty of Agriculture, Cairo University, Cairo, Egypt To cite this article: Farag Malhat , Hany Badawy , Dalia Barakat & Ayman Saber (2013): Determination of etoxazole residues in fruits and vegetables by SPE clean-up and HPLC-DAD, Journal of Environmental Science and Health, Part B: Pesticides, Food Contaminants, and Agricultural Wastes, 48:5, 331-335 To link to this article: http://dx.doi.org/10.1080/03601234.2013.742371 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. -
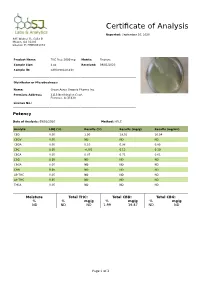
Certificate of Analysis
Certificate of Analysis Reported: September 10, 2020 885 Walnut St. Suite B Macon, GA 31201 License #: PHRS001078 Product Name: THC Free 2000 mg Matrix: Tincture Sample Size: 4 oz Received: 09/01/2020 Sample ID: SAM-090120-218 Distributor or Microbusiness: Name: Green Acres Organic Pharms Inc. Premises Address: 3115 Northington Court Florence, Al 35630 License No.: Potency Date of Analysis: 09/03/2020 Method: HPLC Analyte LOQ (%) Results (%) Results (mg/g) Results (mg/mL) CBD 0.05 1.90 19.01 16.54 CBDV 0.05 ND ND ND CBDA 0.05 0.10 0.98 0.85 CBC 0.05 <LOQ 0.12 0.10 CBCA 0.05 0.07 0.71 0.61 CBG 0.05 ND ND ND CBGA 0.05 ND ND ND CBN 0.05 ND ND ND Δ9-THC 0.05 ND ND ND Δ8-THC 0.05 ND ND ND THCA 0.05 ND ND ND Moisture Total THC: Total CBD: Total CBG: % % mg/g % mg/g % mg/g ND ND ND 1.99 19.87 ND ND Page 1 of 3 Residual Solvents Date of Analysis: 09/09/2020 Method: GC/MS headspace Analyte LOQ (ppm) Results (ppm) Carbon-Tetrachloride 10.00 ND Methanol 10.00 ND Pentane 10.00 ND Ethanol 10.00 ND Acetone 10.00 ND Isopropanol 10.00 ND Acetonitrile 10.00 ND n-Hexane 10.00 ND Chloroform 10.00 ND Benzene 10.00 ND Heptane 10.00 ND Tetrahydrofuran 10.00 ND Toluene 10.00 ND Xylene (meta-, para-) 10.00 ND Xylene (ortho-) 10.00 ND Heavy Metals Date of Analysis: 09/10/2020 Method: ICP/MS Analyte LOQ (ppm) Results (ppm) Cadmium 10.00 ND Lead 10.00 ND Arsenic 10.00 ND Mercury 10.00 ND Pesticides Date of Analysis: 09/03/2020 Method: LC/MS/MS Analyte LOQ (ppb) Results (ppb) Status Aldicarb 100.00 ND Pass Carbofuran 100.00 ND Pass Dimethoate 100.00 ND Pass Ethoprophos -
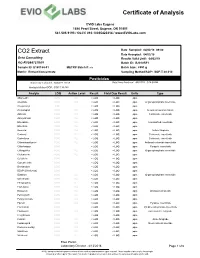
Certificate of Analysis CO2 Extract
Certificate of Analysis EVIO Labs Eugene 1686 Pearl Street, Eugene, OR 97401 541-505-9193 / OLCC 010-10035420314 / www.EVIOLabs.com CO2 Extract Date Sampled: 04/02/18 09:00 Date Accepted: 04/02/18 Octo Consulting Results Valid Until: 04/02/19 AG-R1044757IHH Batch ID: B2018RF1 Sample ID: E180164-01 METRC Batch #: n/a Batch Size: 6991 g Matrix: Extract/Concentrate Sampling Method/SOP: SOP.T.20.010 Pesticides Date/Time Extracted: 04/04/18 09:05 Date/Time Analyzed: 4/6/2018 5:04:52AM Analysis Method/SOP: SOP.T.30.060 Analyte LOQ Action Level Result Field Dup Result Units Type Abamectin 0.250 0.5 < LOQ < LOQ ppm Acephate 0.200 0.4 < LOQ < LOQ ppm Organophosphate insecticide Acequinocyl 1.00 2 < LOQ < LOQ ppm Acetamiprid 0.200 0.2 < LOQ < LOQ ppm Neonicotinoid instecticide Aldicarb 0.200 0.4 < LOQ < LOQ ppm Carbamate insecticide Azoxystrobin 0.200 0.2 < LOQ < LOQ ppm Bifenazate 0.200 0.2 < LOQ < LOQ ppm Unclassified insecticide Bifenthrin 0.200 0.2 < LOQ < LOQ ppm Boscalid 0.200 0.4 < LOQ < LOQ ppm Anilide fungicide Carbaryl 0.200 0.2 < LOQ < LOQ ppm Carbamate insecticide Carbofuran 0.200 0.2 < LOQ < LOQ ppm Carbamate insecticide Chlorantraniliprole 0.200 0.2 < LOQ < LOQ ppm Anthranilic diamide insecticide Chlorfenapyr 0.500 1 < LOQ < LOQ ppm Pyrazole insecticide Chlorpyrifos 0.200 0.2 < LOQ < LOQ ppm Organophosphate insecticide Clofentezine 0.200 0.2 < LOQ < LOQ ppm Cyfluthrin 0.500 1 < LOQ < LOQ ppm Cypermethrin 0.500 1 < LOQ < LOQ ppm Daminozide 0.500 1 < LOQ < LOQ ppm DDVP (Dichlorvos) 0.500 1 < LOQ < LOQ ppm Diazinon 0.200 0.2 < LOQ < -

Pressurized Solvent Extraction with Ethyl Acetate and Liquid Chromatography—Tandem Mass Spectrometry for the Analysis of Selected Conazole Fungicides in Matcha
toxics Article Pressurized Solvent Extraction with Ethyl Acetate and Liquid Chromatography—Tandem Mass Spectrometry for the Analysis of Selected Conazole Fungicides in Matcha Renata Raina-Fulton * and Aisha A. Mohamad Department of Chemistry & Biochemistry, Trace Analysis Facility, University of Regina; 3737 Wascana Parkway, Regina, SK S4S 0A2, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-306-585-4012; Fax: +1-306-337-2409 Received: 30 September 2018; Accepted: 23 October 2018; Published: 25 October 2018 Abstract: The extraction of powdered nutraceuticals is challenging due to the low water content and high concentration of matrix components that can lead to significant matrix effects in liquid chromatography-positive ion electrospray ionization-tandem mass spectrometry (LC-ESI+-MS/MS). In this study we assess the feasibility of using pressurized solvent extraction with ethyl acetate to reduce the co-extraction of polar matrix components. Pigment attributed to chlorophyll was removed with in-cell clean-up utilizing Anasorb 747, Florisil®, and C18. Visible inspection of the extracts showed that pigment was removed from matcha, a powdered green tea sample. Pressurized solvent extraction with in-cell clean-up can be utilized to remove pigments from powdered samples such as nutraceuticals. Average matrix effect of the 32 target analytes that observed mass spectrometric signal suppression or soft MS signal enhancement was −41 ± 19% with the majority of analytes having a protonated molecular ion with m/z of 250 to 412. As generally moderate signal suppression was observed for conazole fungicides and structurally related compounds analyzed by LC-ESI+-MS/MS, it is recommended that matrix matched or standard addition calibration is used for quantitation. -

Standards for Cannabis Testing
Standards for Cannabis Testing Cannabis Standards AccuStandard offers a comprehensive list of standards for testing the quality and safety of cannabis and hemp products for both medicinal and recreational purposes. Standards for testing includes pesticides, residual solvents, terpenes (for strain and flavor profiling), heavy metals, and active cannabinoid compounds. Visit our website for the latest cannabis updates https://www.accustandard.com/cannabis-standards Contents Cannabinoids 1 Cannabis Terpenes 1 Hemp 1 Cannabis State Methods 2-4 California Residual Pesticides & Solvents 2 Colorado Residual Pesticides 2 Florida Pesticides 2 Maryland Residual Pesticides 3 Massachusetts Residual Pesticides 3 Nevada Pesticides 3 Oregon Residual Pesticides & Solvents 3-4 Pennsylvania Pesticides 4 Heavy Metals As, Cd, Cr, Pb, Hg, Ni, Tl 4 Canada Residual Pesticides & Solvents 5 17034, 17025, and 9001:2015 © AccuStandard, Inc. 2021 AccuStandard is an ISO accredited Reference Material Producer Cannabis Standards Cannabinoids Cannabis Terpenes SOLUTIONS at 1000 µg/mL in PT Methanol SOLUTIONS at 100 µg/mL in PT Methanol Compound CAS Cat. No. Unit Compound CAS Cat. No. Unit Cannabidiol (CBD) 13956-29-1 CP-CBD-01S 1 mL (−)-alpha-Bisabolol 23089-26-1 CP-TER-001S 1 mL Delta-8-Tetrahydrocannabinol 5957-75-5 CP-8-THC-01S 1 mL beta-Pinene 18172-67-3 CP-TER-002S 1 mL (THC-8) (−)-Borneol 464-45-9 CP-TER-003S 1 mL Delta-9-Tetrahydrocannabinol 1972-08-3 CP-9-THC-01S 1 mL (−)-Caryophyllene oxide 1139-30-6 CP-TER-004S 1 mL (THC-9) (−)-Guaiol 489-86-1 CP-TER-005S 1 mL Delta-9-Tetrahydrocannabinolic 23978-85-0 CP-THCA-A-01S 1 mL (−)-Isopulegol 89-79-1 CP-TER-006S 1 mL acid A (THCA-A) (+)-Borneol 464-43-7 CP-TER-007S 1 mL Cannabigerol (CBG) 25654-31-3 CP-CBG-01S 1 mL (+)-Fenchone 4695-62-9 CP-TER-008S 1 mL Cannabichromene (CBC) 20675-51-8 CP-CBC-01S 1 mL Eucalyptol 470-82-6 CP-TER-009S 1 mL alpha-Humulene 6753-98-6 CP-TER-010S 1 mL alpha-Pinene 80-56-8 CP-TER-011S 1 mL Restrictions may apply, contact us for information. -
Guide for Pesticide Solubility
Guide for Pesticide Solubility spex.com Connect with us Phone: +1.732.549.7144 • +1.800.LAB.SPEX Spex CertiPrep is an Fax: +1.732.603.9647 Antylia Scientific company. [email protected] Find out more at antylia.com. 4772AY Guide for Pesticide Solubility Chemical pesticides have become an integral part of the agricultural toolbox, offering protection to crops from destructive pests. However, some unfortunate side effects of their use include potential leaching of these, oftentimes, harmful chemicals into the environment and their eventual presence in the human food chain. As a result, pesticide residue analysis has become a critical testing process for many different types of laboratories. Unfortunately, pesticide residue testing is a long, expensive and complicated process, covering hundreds of different compounds, made even more daunting by the plethora of varying pesticide types that sometimes require different testing techniques for identification. Fortunately, as the leader in HPLC, GC, LC/MS and GC/MS pesticide CRMs, Spex CertiPrep is happy to assist all of our customers with all of their pesticide CRM needs. For your convenience we have designed a pesticide residue testing kit which includes 144 of the most commonly analyzed pesticides per EPA, AOAC, FDA and other international testing methods. The kit is structured to maximize stability and solubility while minimizing unwanted analyte interaction and interference; enjoy shorter calibration times, fewer injections and money savings, as compared to purchasing individual pesticide standards. As a companion piece to this new product, we have assembled this solubility guide, which covers all 144 pesticides included in the kit (Spex CertiPrep part number SPXPR-KIT). -
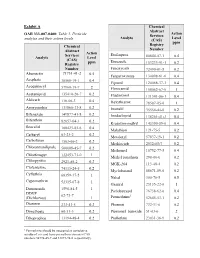
Exhibit a OAR 333-007-0400: Table 3. Pesticide Analytes and Their
Exhibit A Chemical Abstract Action OAR 333-007-0400: Table 3. Pesticide Services Analyte Level analytes and their action levels (CAS) ppm Chemical Registry Abstract Number Action Services Etofenprox Analyte Level 80844-07-1 0.4 (CAS) ppm Etoxazole Registry 153233-91-1 0.2 Number Fenoxycarb 72490-01-8 0.2 Abamectin 71751-41-2 0.5 Fenpyroximate 134098-61-6 0.4 Acephate 30560-19-1 0.4 Fipronil 120068-37-3 0.4 Acequinocyl 57960-19-7 2 Flonicamid 158062-67-0 1 Acetamiprid 135410-20-7 0.2 Fludioxonil 131341-86-1 0.4 Aldicarb 116-06-3 0.4 Hexythiazox 78587-05-0 1 Azoxystrobin 131860-33-8 0.2 Imazalil 35554-44-0 0.2 Bifenazate 149877-41-8 0.2 Imidacloprid 138261-41-3 0.4 Bifenthrin 82657-04-3 0.2 Kresoxim-methyl 143390-89-0 0.4 Boscalid 188425-85-6 0.4 Malathion 121-75-5 0.2 Carbaryl 63-25-2 0.2 Metalaxyl 57837-19-1 0.2 Carbofuran 1563-66-2 0.2 Methiocarb 2032-65-7 0.2 Chlorantraniliprole 500008-45-7 0.2 Methomyl 16752-77-5 0.4 Chlorfenapyr 122453-73-0 1 Methyl parathion 298-00-0 0.2 Chlorpyrifos 2921-88-2 0.2 MGK-264 113-48-4 0.2 Clofentezine 74115-24-5 0.2 Myclobutanil 88671-89-0 0.2 Cyfluthrin 68359-37-5 1 Naled 300-76-5 0.5 Cypermethrin 52315-07-8 1 Oxamyl 23135-22-0 1 Daminozide 1596-84-5 1 Paclobutrazol 76738-62-0 0.4 DDVP 62-73-7 1 (Dichlorvos) 1 Permethrins 52645-53-1 0.2 Diazinon 333-41-5 0.2 Phosmet 732-11-6 0.2 Dimethoate 60-51-5 0.2 Piperonyl_butoxide 51-03-6 2 Ethoprophos 13194-48-4 0.2 Prallethrin 23031-36-9 0.2 1 Permethrins should be measured as cumulative residue of cis- and trans-permethrin isomers (CAS numbers 54774-45-7 and 51877-74-8 respectively). -

Use of High Throughput Assays and Computational Tools; Endocrine Disruptor Screening Program; Notice of Availability and Opportunity for Comment’’
NRDC EPA-HQ-OPPT-2015-0305 August, 2015 Comments from the Natural Resources Defense Council On The Document Titled, ‘‘Use of High Throughput Assays and Computational Tools; Endocrine Disruptor Screening Program; Notice of Availability and Opportunity for Comment’’ To the U.S. Environmental Protection Agency Docket No. EPA-HQ-OPPT-2015-0305 August 18, 2015 Background The Natural Resources Defense Council ("NRDC") is a national, non-profit environmental organization of lawyers, scientists, and other professionals. NRDC presents these comments on behalf of our 1.4 million members and online activists. NRDC does not have any financial interest in the topic of these comments. The endocrine system utilizes highly complex, tightly controlled molecular processes for its optimal functioning in the body. The proper balance of hormones can be synchronized in a variety of ways (including direct protein binding, epigenetic alterations, gene activation and silencing), and is essential across the entirety of the life course. Small changes in the perfectly orchestrated symphony of hormone levels can severely disrupt the harmony necessary for critical windows of development (e.g., fetal development, infanthood, childhood, and adolescence), leaving the body vulnerable to a host of negative health outcomes (such as diabetes, cancer, obesity, and reproductive dysfunction). The last decade has seen an exponential increase in the development of computational, biological, and chemical tools capable of increasing both the number of chemicals analyzed and the pace of chemical toxicity evaluation. These tools, including the EPA Toxicity Forecaster (ToxCast™) and the National Institute of Environmental Health Sciences (NIEHS) Tox21 platforms, have the potential to rapidly generate molecular and cellular data for thousands of chemicals at once, and provide an additional stream of useful information that can aide in regulatory decision-making. -

Reports of Adverse Health Effects in Manufacturing Plant Personnel Or in Operators and Workers Exposed to Etoxazole Formulations During Their Use
Etoxazole 209 5.13 ETOXAZOLE (241) TOXICOLOGY Etoxazole is the International Organization for Standardization (ISO)–approved name for (RS)-5-tert- butyl-2-[2-(2,6-difluorophenyl)-4,5-dihydro-1,3-oxazol-4-yl]phenetole (International Union of Pure and Applied Chemistry [IUPAC]), with Chemical Abstracts Service (CAS) No. 153233-91-1. Etoxazole is a new acaricide that belongs to the diphenyloxazole class of miticides/ovicides, possibly acting by inhibiting chitin biosynthesis and causing adults to lay sterile eggs. Etoxazole has not been evaluated previously by the Joint FAO/WHO Meeting on Pesticide Residues (JMPR) and was reviewed at the present Meeting at the request of the Codex Committee on Pesticide Residues (CCPR). All the pivotal studies contained certificates of compliance with good laboratory practice (GLP). Biochemical aspects The absorption, distribution, metabolism and excretion of etoxazole were investigated in rats. 14C- labelled etoxazole was rapidly but only moderately absorbed from the gastrointestinal tract of rats following oral dosing. Maximum concentrations of radioactivity in plasma were observed within 2– 4 h of dosing for the low dose group (5 mg/kg body weight [bw]) and within 4–6 h for the high dose group (500 mg/kg bw). At the low dose, the degree of absorption in males (50–54%) was less than that in females (63–70%), but there were no major sex-related differences in the pattern of excretion. Saturation of absorption occurred at a high dose (500 mg/kg bw per day), with less than 30% absorbed. Faecal excretion was the primary route of elimination, and excretion was essentially complete within 120 h after dosing. -
Etoxazole 1133
Etoxazole 1133 ETOXAZOLE (241) First draft prepared by Mr Makoto Irie, Ministry of Agriculture, Forestry and Fisheries, Tokyo, Japan EXPLANATION Etoxazole is an acaricide (miticide/ovicide) belonging to the diphenyloxazoline group of chemicals. It controls mites through inhibition of chitin biosynthesis and by causing adults to lay sterile eggs. At the Forty-first session of the CCPR (2009), etoxazole was scheduled for the evaluation as a new compound by 2010 JMPR. The Meeting received information on identity, animal and plant metabolism, environment fate in soil, rotational crops, analytical methods, storage stability, use patterns, supervised trials, farm animal feeding studies and fates of residues in processing IDENTITY Common name Etoxazole Chemical name IUPAC: (RS)-5-tert-butyl-2-[2-(2,6-difluorophenyl)-4,5-dihydro-1,3-oxazol-4-yl] phenetole CAS: 2-(2,6-difluorophenyl-4-[4-(1,1-dimethylethyl)-2-ethoxyphenyl]-4,5- dihydrooxazole CAS number: 153233-91-1 Synonyms: V-1283, S-1283, YI-5301, SCAL-5001 Structural formula: F OC H 2 CH 3 N (C H 3 ) 3 C O F Molecular formula: C21H23F2NO2 Molecular weight: 359.4 PHYSICAL AND CHEMICAL PROPERTIES Pure active ingredient Property Results Reference Appearance White N 9.5/ with 90% reflectance free flowing Betteley, 1997 (SKP- crystalline powder 0005) Odour No obvious odour Betteley, 1997 (SKP- 0005) Melting point 101.5–102.5 °C Betteley, 1997 (SKP- 0005) 1134 Etoxazole Property Results Reference Relative density 1.2389 kg/m3 Betteley, 1997 (SKP- 0005) Vapour pressure 7.0 10-6 Pa at 25 °C Betteley, 1997 (SKP- 0005) Volatility (Henry’s law constant) Henry’s law constant (calculated) 3.6 10-2 Pa m3/mole Betteley, 1997 (SKP- at 20–25 °C 0005) Solubility in water 3.99 10-5 g/L in distilled water at 10 °C Betteley, 1997 (SKP- 7.04 10-5 g/L in distilled water at 20 °C 0005) 6.69 10-5 g/L in distilled water at 30 °C The effect of pH was not determined as the test material has no ionisable groups or dissociation constant.