The Fauna of King Island
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Commonwealth Listing Advice on Alpine Sphagnum Bogs And
Advice to the Minister for the Environment, Heritage and the Arts from the Threatened Species Scientific Committee (the Committee) on Amendments to the List of Ecological Communities under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) 1. Summary of conservation assessment by the Committee This advice follows the assessment of information provided by a nomination to list the Alpine Bog Community as a threatened ecological community. The nomination was made available for public exhibition and comment for a period of two months. The Committee had regard to all public and expert comments that were relevant to the survival of the ecological community. The Committee judges that the ecological community has been demonstrated to have met sufficient elements of: • Criterion 2 to make it eligible for listing as endangered; • Criterion 3 to make it eligible for listing as endangered; and • Criterion 4 to make it eligible for listing as endangered. 2. Name of the ecological community A nomination was received for the Alpine Bog Community. Alpine bogs are commonly found in the vicinity of alpine fens, and have been identified as being dependent on these (VSAC, 1991a). In order to make identification easier in the field, to recognise the importance of Sphagnum species to alpine bogs, and to acknowledge the interdependency of alpine bogs, fens and the natural drainage lines that connect them, the Committee recommends that the name of the ecological community be changed to the “Alpine Sphagnum Bogs and Associated Fens” ecological community. 3. Description The Alpine Sphagnum Bogs and Associated Fens ecological community generally has sharp boundaries and is easily delineated from other alpine vegetation communities. -

Vegetation Benchmarks Rainforest and Related Scrub
Vegetation Benchmarks Rainforest and related scrub Eucryphia lucida Vegetation Condition Benchmarks version 1 Rainforest and Related Scrub RPW Athrotaxis cupressoides open woodland: Sphagnum peatland facies Community Description: Athrotaxis cupressoides (5–8 m) forms small woodland patches or appears as copses and scattered small trees. On the Central Plateau (and other dolerite areas such as Mount Field), broad poorly– drained valleys and small glacial depressions may contain scattered A. cupressoides trees and copses over Sphagnum cristatum bogs. In the treeless gaps, Sphagnum cristatum is usually overgrown by a combination of any of Richea scoparia, R. gunnii, Baloskion australe, Epacris gunnii and Gleichenia alpina. This is one of three benchmarks available for assessing the condition of RPW. This is the appropriate benchmark to use in assessing the condition of the Sphagnum facies of the listed Athrotaxis cupressoides open woodland community (Schedule 3A, Nature Conservation Act 2002). Benchmarks: Length Component Cover % Height (m) DBH (cm) #/ha (m)/0.1 ha Canopy 10% - - - Large Trees - 6 20 5 Organic Litter 10% - Logs ≥ 10 - 2 Large Logs ≥ 10 Recruitment Continuous Understorey Life Forms LF code # Spp Cover % Immature tree IT 1 1 Medium shrub/small shrub S 3 30 Medium sedge/rush/sagg/lily MSR 2 10 Ground fern GF 1 1 Mosses and Lichens ML 1 70 Total 5 8 Last reviewed – 2 November 2016 Tasmanian Vegetation Monitoring and Mapping Program Department of Primary Industries, Parks, Water and Environment http://www.dpipwe.tas.gov.au/tasveg RPW Athrotaxis cupressoides open woodland: Sphagnum facies Species lists: Canopy Tree Species Common Name Notes Athrotaxis cupressoides pencil pine Present as a sparse canopy Typical Understorey Species * Common Name LF Code Epacris gunnii coral heath S Richea scoparia scoparia S Richea gunnii bog candleheath S Astelia alpina pineapple grass MSR Baloskion australe southern cordrush MSR Gleichenia alpina dwarf coralfern GF Sphagnum cristatum sphagnum ML *This list is provided as a guide only. -

Flinders Island Tourism and Business Inc. /Visitflindersisland
Flinders Island Tourism and Business Inc. www.visitflindersisland.com.au /visitflindersisland @visitflindersisland A submission to the Rural & Regional Affairs and Transport References Committee The operation, regulation and funding of air route service delivery to rural, regional and remote communities with particular reference to: Background The Furneaux Islands consist of 52 islands with Cape Barren and Flinders Island being the largest. The local Government resident population at the 2016 census was 906 and rose 16 % between the last two censuses. The Flinders Island Tourism & Business Inc. (FITBI)represents 70 members across retail, tourism, fishing and agriculture. It plays a key role in developing the visitor economy through marketing to potential visitors as well as attracting residents to the island. In 2016, FITBI launched a four-year marketing program. The Flinders Island Airport at Whitemark is the gateway to the island. It has been owned by the Flinders Council since hand over by the Commonwealth Government in the early 1990’s. Being a remote a community the airport is a critical to the island from a social and economic point of view. It’s the key connection to Tasmania (Launceston) and Victoria. The local residents must use air transport be that for health or family reasons. The high cost of the air service impacts on the cost of living as well as discouraging visitors to visit the island. It is particularly hard for low income families. The only access via sea is with the barge, Matthew Flinders 11, operated by Furneaux Freight out of Bridport. This vessel has very basic facilities for passengers and takes 8hours one way. -
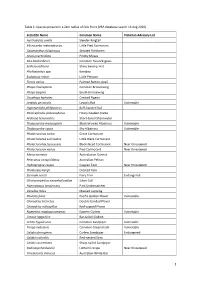
Scientific Name Common Name Victorian A
Table 1: Species present in a 2km radius of Crib Point (VBA database search 13 Aug 2020) Scientific Name Common Name Victorian Advisory List Austrolestes analis Slender Ringtail Microcarbo melanoleucos Little Pied Cormorant Calamanthus fuliginosus Striated Fieldwren Acacia verticillata Prickly Moses Poa labillardierei Common Tussock-grass Selliera radicans Shiny Swamp-mat Phyllostachys spp. Bamboo Eudyptula minor Little Penguin Turnix varius Painted Button-quail Phaps chalcoptera Common Bronzewing Phaps elegans Brush Bronzewing Ocyphaps lophotes Crested Pigeon Lewinia pectoralis Lewin's Rail Vulnerable Hypotaenidia philippensis Buff-banded Rail Poliocephalus poliocephalus Hoary-headed Grebe Ardenna tenuirostris Short-tailed Shearwater Thalassarche melanophris Black-browed Albatross Vulnerable Thalassarche cauta Shy Albatross Vulnerable Phalacrocorax carbo Great Cormorant Phalacrocorax sulcirostris Little Black Cormorant Phalacrocorax fuscescens Black-faced Cormorant Near threatened Phalacrocorax varius Pied Cormorant Near threatened Morus serrator Australasian Gannet Pelecanus conspicillatus Australian Pelican Hydroprogne caspia Caspian Tern Near threatened Thalasseus bergii Crested Tern Sternula nereis Fairy Tern Endangered Chroicocephalus novaehollandiae Silver Gull Haematopus longirostris Pied Oystercatcher Vanellus miles Masked Lapwing Pluvialis fulva Pacific Golden Plover Vulnerable Charadrius bicinctus Double-banded Plover Charadrius ruficapillus Red-capped Plover Numenius madagascariensis Eastern Curlew Vulnerable Limosa lapponica -
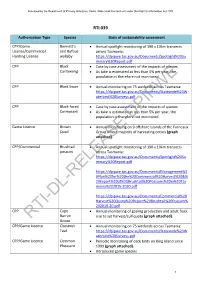
Rti-Dl-Release-Dpipwe
Assessed by the Department of Primary Industries, Parks, Water and Environment under the Right to Information Act 2009 RTI 039 Authorisation Type Species Basis of sustainability assessment CPP/Game Bennett’s • Annual spotlight monitoring of 190 x 10km transects Licence/Commercial and Rufous across Tasmania: Hunting Licence wallaby https://dpipwe.tas.gov.au/Documents/Spotlight%20Su mmary%20Report.pdf CPP Black • Case by case assessment of the impacts of species Currawong • As take is estimated at less than 5% per year, the population is therefore not monitored. CPP Black Swan • Annual monitoring on 75 wetlands across Tasmania: https://dpipwe.tas.gov.au/Documents/Statewide%20W aterbird%20Surveys.pdf CPP Black-faced • Case by case assessment of the impacts of species Cormorant • As take is estimated at less than 5% per year, the population is therefore not monitored. Game Licence Brown • Annual monitoring on 9 offshore islands of the Furneaux Quail Group where majority of harvesting occurs (graph attached). CPP/Commercial Brushtail • Annual spotlight monitoring of 190 x 10km transects possum across Tasmania: https://dpipwe.tas.gov.au/Documents/Spotlight%20Su mmary%20Report.pdf https://dpipwe.tas.gov.au/Documents/Management%2 0Plan%20for%20the%20Commercial%20Harvest%20&% 20Export%20of%20Brushtail%20Possums%20in%20Tas mania%202015-2020.pdf https://dpipwe.tas.gov.au/Documents/Commercial%20 Harvest%20Quota%20Report%20Brushtail%20Possum% 202019-20.pdf CPP Cape • Annual monitoring of gosling production and adult flock Barren size to set harvest/cull quota (graph attached). RTI-DL-RELEASE-DPIPWEGoose CPP/Game Licence Chestnut • Annual monitoring on 75 wetlands across Tasmania: Teal https://dpipwe.tas.gov.au/Documents/Statewide%20W aterbird%20Surveys.pdf CPP/Game Licence Common • Periodic monitoring of cock birds on King Island since Pheasant 1999 (graph attached). -

National Recovery Plan for the Regent Honeyeater (Anthochaera Phrygia)
National Recovery Plan for the Regent Honeyeater (Anthochaera phrygia) April 2016 1 The Species Profile and Threats Database pages linked to this recovery plan is obtainable from: http://www.environment.gov.au/cgi-bin/sprat/public/sprat.pl © Copyright Commonwealth of Australia, 2016. The National Recovery Plan for the Regent Honeyeater (Anthochaera phrygia) is licensed by the Commonwealth of Australia for use under a Creative Commons Attribution 4.0 International licence with the exception of the Coat of Arms of the Commonwealth of Australia, the logo of the agency responsible for publishing the report, content supplied by third parties, and any images depicting people. For licence conditions see: https://creativecommons.org/licenses/by/4.0/. This report should be attributed as ‘National Recovery Plan for the Regent Honeyeater (Anthochaera phrygia), Commonwealth of Australia 2016’. The Commonwealth of Australia has made all reasonable efforts to identify content supplied by third parties using the following format ‘© Copyright, [name of third party] ’. Disclaimer While reasonable efforts have been made to ensure that the contents of this publication are factually correct, the Commonwealth does not accept responsibility for the accuracy or completeness of the contents, and shall not be liable for any loss or damage that may be occasioned directly or indirectly through the use of, or reliance on, the contents of this publication. Image credits Front Cover: Regent honeyeaters in the Capertee Valley, NSW. (© Copyright, Dean Ingwersen). 2 -
Macquarie River Bird Trail
Bird Watching Trail Guide Acknowledgements RiverSmart Australia Limited would like to thank the following for their assistance in making this trail and publication a reality. Tim and Janis Hosking, and the other members of the Dubbo Field Naturalists and Conservation Society, who assisted with technical information about the various sites, the bird list and with some of the photos. Thanks also to Jim Dutton for providing bird list details for the Burrendong Arboretum. Photographers. Photographs were kindly provided by Brian O’Leary, Neil Zoglauer, Julian Robinson, Lisa Minner, Debbie Love, Tim Hosking, Dione Carter, Dan Giselsson, Tim Ralph and Bill Phillips. This project received financial support from the Australian Bird Environment Foundation of Sacred kingfisher photo: Dan Giselsson BirdLife Australia. Thanks to Warren Shire Council, Sarah Derrett and Ashley Wielinga in particular, for their assistance in relation to the Tiger Bay site. Thanks also to Philippa Lawrence, Sprout Design and Mapping Services Australia. THE MACQuarIE RIVER TraILS First published 2014 The Macquarie valley, in the heart of NSW is one of the The preparation of this guide was coordinated by the not-for-profit organisation Riversmart State’s — and indeed Australia’s — best kept secrets, until now. Australia Ltd. Please consider making a tax deductible donation to our blue bucket fund so we can keep doing our work in the interests of healthy and sustainable rivers. Macquarie River Trails (www.rivertrails.com.au), launched in late 2011, is designed to let you explore the many attractions www.riversmart.org.au and wonders of this rich farming region, one that is blessed See outside back cover for more about our work with a vibrant river, the iconic Maquarie Marshes, friendly people and a laid back lifestyle. -

Abrolhos Painted Button-Quail (Turnix Varius Scintillans) Interim Recovery Plan
Abrolhos Painted Button-Quail (Turnix varius scintillans) Interim Recovery Plan Wildlife Management Program No. 63 Western Australia Department of Biodiversity, Conservation and Attractions May 2018 Wildlife Management Program No. 63 Abrolhos Painted Button-Quail (Turnix varius scintillans) Interim Recovery Plan Western Australia Department of Biodiversity, Conservation and Attractions Locked Bag 104, Bentley Delivery Centre, Western Australia 6983 Foreword Recovery plans are developed within the framework laid down in the Department of Biodiversity, Conservation and Attractions Corporate Policy Statement No. 35 (Parks and Wildlife, 2015b) and Corporate Guideline No. 36 (Parks and Wildlife, 2015a). Interim recovery plans outline the recovery actions that are needed to urgently address those threatening processes most affecting the ongoing survival of threatened taxa or ecological communities, and begin the recovery process. The attainment of objectives and the provision of funds necessary to implement actions are subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. This interim recovery plan was approved by the Department of Biodiversity, Conservation and Attractions, Western Australia. Approved interim recovery plans are subject to modification as dictated by new findings, changes in status of the taxon or ecological community, and the completion of recovery actions. Information in this interim recovery plan was accurate as of May 2018. Interim recovery plan preparation: -

Great Australian Bight BP Oil Drilling Project
Submission to Senate Inquiry: Great Australian Bight BP Oil Drilling Project: Potential Impacts on Matters of National Environmental Significance within Modelled Oil Spill Impact Areas (Summer and Winter 2A Model Scenarios) Prepared by Dr David Ellis (BSc Hons PhD; Ecologist, Environmental Consultant and Founder at Stepping Stones Ecological Services) March 27, 2016 Table of Contents Table of Contents ..................................................................................................... 2 Executive Summary ................................................................................................ 4 Summer Oil Spill Scenario Key Findings ................................................................. 5 Winter Oil Spill Scenario Key Findings ................................................................... 7 Threatened Species Conservation Status Summary ........................................... 8 International Migratory Bird Agreements ............................................................. 8 Introduction ............................................................................................................ 11 Methods .................................................................................................................... 12 Protected Matters Search Tool Database Search and Criteria for Oil-Spill Model Selection ............................................................................................................. 12 Criteria for Inclusion/Exclusion of Threatened, Migratory and Marine -

The Relationships of the Starlings (Sturnidae: Sturnini) and the Mockingbirds (Sturnidae: Mimini)
THE RELATIONSHIPS OF THE STARLINGS (STURNIDAE: STURNINI) AND THE MOCKINGBIRDS (STURNIDAE: MIMINI) CHARLESG. SIBLEYAND JON E. AHLQUIST Departmentof Biologyand PeabodyMuseum of Natural History,Yale University, New Haven, Connecticut 06511 USA ABSTRACT.--OldWorld starlingshave been thought to be related to crowsand their allies, to weaverbirds, or to New World troupials. New World mockingbirdsand thrashershave usually been placed near the thrushesand/or wrens. DNA-DNA hybridization data indi- cated that starlingsand mockingbirdsare more closelyrelated to each other than either is to any other living taxon. Some avian systematistsdoubted this conclusion.Therefore, a more extensiveDNA hybridizationstudy was conducted,and a successfulsearch was made for other evidence of the relationshipbetween starlingsand mockingbirds.The resultssup- port our original conclusionthat the two groupsdiverged from a commonancestor in the late Oligoceneor early Miocene, about 23-28 million yearsago, and that their relationship may be expressedin our passerineclassification, based on DNA comparisons,by placing them as sistertribes in the Family Sturnidae,Superfamily Turdoidea, Parvorder Muscicapae, Suborder Passeres.Their next nearest relatives are the members of the Turdidae, including the typical thrushes,erithacine chats,and muscicapineflycatchers. Received 15 March 1983, acceptedI November1983. STARLINGS are confined to the Old World, dine thrushesinclude Turdus,Catharus, Hylocich- mockingbirdsand thrashersto the New World. la, Zootheraand Myadestes.d) Cinclusis -

The Mcguire Center for Lepidoptera and Biodiversity
Supplemental Information All specimens used within this study are housed in: the McGuire Center for Lepidoptera and Biodiversity (MGCL) at the Florida Museum of Natural History, Gainesville, USA (FLMNH); the University of Maryland, College Park, USA (UMD); the Muséum national d’Histoire naturelle in Paris, France (MNHN); and the Australian National Insect Collection in Canberra, Australia (ANIC). Methods DNA extraction protocol of dried museum specimens (detailed instructions) Prior to tissue sampling, dried (pinned or papered) specimens were assigned MGCL barcodes, photographed, and their labels digitized. Abdomens were then removed using sterile forceps, cleaned with 100% ethanol between each sample, and the remaining specimens were returned to their respective trays within the MGCL collections. Abdomens were placed in 1.5 mL microcentrifuge tubes with the apex of the abdomen in the conical end of the tube. For larger abdomens, 5 mL microcentrifuge tubes or larger were utilized. A solution of proteinase K (Qiagen Cat #19133) and genomic lysis buffer (OmniPrep Genomic DNA Extraction Kit) in a 1:50 ratio was added to each abdomen containing tube, sufficient to cover the abdomen (typically either 300 µL or 500 µL) - similar to the concept used in Hundsdoerfer & Kitching (1). Ratios of 1:10 and 1:25 were utilized for low quality or rare specimens. Low quality specimens were defined as having little visible tissue inside of the abdomen, mold/fungi growth, or smell of bacterial decay. Samples were incubated overnight (12-18 hours) in a dry air oven at 56°C. Importantly, we also adjusted the ratio depending on the tissue type, i.e., increasing the ratio for particularly large or egg-containing abdomens. -

1 General Introduction
1 General Introduction If the karate-ka (student) shall walk the true path, first he will cast aside all preference. Tatsuo Shimabuku, Grand Master of Isshin-ryu Karate 1.1 The Importance of Insects ~30% of the plants we grow for food and materials. Because of their great numbers and diversity, insects Insects transmit some of these pathogens. While have a considerable impact on human life and indus- weeds can often reduce pest attack, they can also try, particularly away from cities and in the tropics. harbour the pest’s enemies or provide alternative On the positive side they form a large and irreplace- resources for the pest itself. Then in storage, insects, able part of the ecosystem, especially as pollinators mites, rodents and fungi cause a further 30% loss. of fruit and vegetable crops and, of course, many Apart from such biotic damage, severe physical con- wild plants (Section 8.2.1). They also have a place ditions such as drought, storms and flooding cause in soil formation (Section 8.2.4) and are being used additional losses. For example, under ideal field increasingly in ‘greener’ methods of pest control. conditions new wheat varieties (e.g. Agnote and Biological control using insects as predators and Humber) would give yields of ~16 tonnes/ha, but parasites of pest insects has been developed in the produce typically about half this under good hus- West for over a century, and much longer in China. bandry. Pre-harvest destruction due only to insects More recently integrated pest management (IPM) is 10–13% (Pimentel et al., 1984; Thacker, 2002).