PDF (Volume 2)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VERRE À Recycler
CABOURG 8 PAGES_Mise en page 1 21/12/16 15:24 Page1 cabourg GUIDE DUTRI normandie cabourg 2017 pays d’auge NOUVEAU calendrier des collectes pour les particuliers cOllecte de vOs déchets RAPPEL Ordures ménagères hors saison : lundi, vendredi juillet-août : lundi, mercredi, vendredi les sacs et les bacs doi- vent être sortis entre 19h sacs jaunes jeudi (la veille de la collecte) et 5h du matin (le jour de encOmbrants jeudis 2 février, 4 mai, 14 septembre, 23 novembre 2017 la collecte). pour un cadre de vie agréable pour tous, déchets verts tous les lundis du 13 mars au 13 novembre 2017 les bacs roulants sont à sapins de nOël lundi 9 janvier 2017 ranger le plus vite possi- ble dès qu’ils sont vidés. les collectes sont assurées les jours fériés sauf le 25 décembre 2017 et le 1er janvier 2018 CABOURG 8 PAGES_Mise en page 1 21/12/16 15:24 Page2 mémo Non recyclés tri uniQuement bOuteilles et flacOns en plastiQue a jeter dans votre poubelle ordinaire ou à déposer à la déchetterie emballages métalliQues emballages en cartOn EMBALLAGES Non recyclés ET PAPIERS à recycler tOus les papiers journaux, magazines, publicités, catalogues, livres, cahiers, briQues alimentaires enveloppes, courriers... a jeter dans votre poubelle ordinaire EMBALLAGES EN VERRE à recycler bOuteilles en verre pOts et bOcaux en verre CABOURG 8 PAGES_Mise en page 1 21/12/16 15:24 Page3 cOmment alléger vOs pOubelles ? 7 astuces faciles 4 portez vos textiles usagés au conteneur « textiles » (voir localisation compostez ! pages 6 et 7). 1 triez les emballages recycla- 5 bles (papiers, cartons, bouteilles et déposez à la déchetterie vos flacons en plastiques, briques ali- huiles de friture, vos pots de pein- mentaires, emballages métalliques) ture, vos gravats, vos encombrants… dans le sac ou le bac jaune. -

Paris and Normandy River Cruise
Paris and Normandy River Cruise Through the Eyes of a Woman! April 22 - May 2, 2019 WO MEN OF N EBRASKA Travel Solo Tog ether Dear Women of Nebraska, Join me on a once-in-a-lifetime journey to Northern France! Join our exceptional Paris-Normandy river cruise on board the deluxe AmaLyra of AmaWaterways along the Seine River and through the heart of Normandy. With a capacity of 74 outside staterooms only, this cruise gives us the private feeling we are looking for. Our 11-day tour begins in Paris, the City of Light, with its iconic landmarks, aristocratic lifestyle, romantic ardor, architectural splendor, animated sidewalk cafes and, world-class fashion and shopping. Ahead of us awaits Monet’s Gardens in Giverny and Rouen’s Cathedral of Notre Dame. The charming harbor town of Honfleur will inspire you the same way as they inspired the great Impressionists. We will get to see some of these very same places and landmarks that the Impressionist Masters captured on canvas at the Musée d’Orsay, during our stay in Paris. For an inspiration of a different kind, we travel the “Routes des Abbayes” (Route of the Historic Norman Abbeys), visiting some of the most magnificent monasteries, and to the unforgettable beaches of Normandy where Allied forces landed during WWII’s D-Day invasion. We reflect on Journal Star Destinations the “longest day” and honor the sacrifices made in changing history not once, but twice. We will relive the grandeur of royalty at Château Malmaison, the former home of Napoleon and Josephine Bonaparte, and at Chateau de Bizy, once referred to as “the Versailles of Normandy.” Blend a passion for the good life with culture, art, architecture and timeless landscapes, and you have Northern France! Come, join me! Solo or two-by-two! Sincerely, Sally Dunham Ambassador, Women of Nebraska Call Executive Travel’s Group Department today at 402-435-8888. -
1 11 RD513 Page 151 a 237.Pdf
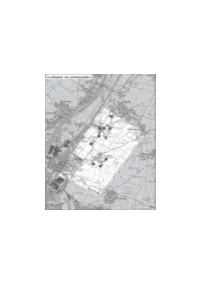
La construction d'un paysage Château d'eau Le patrimoine végétal Alignement Château d'eau Boisements RANVILLE BENOUVILLE 3 Les vues principales du paysage R 22 D RD 3 7 Dégagements visuels sur le grand paysage Eléments de cadrage des vues BLAINVILLE-SUR-ORNE sur le paysage de la zone 15 5 Repères visuels D R Ligne Haute Tension Château d'eau, usines, silos Centrale électrique 02 4 Cimenterie HEROUVILLETTE D R Limite de commune Château d'eau Limite de la zone d'étude 3 2 2 D Usine R 13 RD 5 Ste-Honorine la Chardronnette ESCOVILLE Château d'eau Silo 28 2 D Usine d'incinération R R D 226 Antenne radio Château d'eau CUVERVILLE Echelle : 1 / 25 000ème Château d'eau Scan25©IGN 638-30/PL/02.02.05 Pièce 5 - Etude d’impact D - Le paysage de village rurbanisé B - Les vues principales de ce paysage On note sur le site l’existence de villages autrefois ruraux qui sont maintenant sujets à une La zone d’étude comporte de multiples dégagements visuels. Ceux-ci prennent plus ou moins importante rurbanisation. Ces villages ne semblent plus liés à la forme d’occupation agricole du de champ par les effets de cadrages réalisés par les implantations bâties et les ensembles territoire. boisés. Par ailleurs, la topographie permet par endroits de bénéficier d’un effet de promontoire, une vue se dégage alors vers le grand paysage. Les lointains sont composés par l’estuaire de Le hameau de « Sainte-Honorine-la-Chardronnette » et le bourg de la commune l’Orne, voire même de l’ensemble de la vallée avec ses coteaux. -

Falaise Du Cap Romain
Réserve Naturelle FALAISE DU CAP ROMAIN Plan de gestion 2020-2024 Section A - Diagnostic 1 Plan de gestion 2020-2024 de la RNN Falaise du Cap Romain 29 janvier 2021 Illustration de couverture : vue de la falaise du Cap Romain, éponge fossile (Platychonia magna), coupe géologique du Cap Romain Rédaction : Anne-Lise GIOMMI, conservatrice de la RNN Falaise du Cap Romain Relecture : Florence Magliocca (DREAL Normandie), Delphine Boutard (Département du Calvados) Géologie : Jacques Avoine et Olivier Dugué (APGN et Université de Caen), Jean-Pierre Camuzard et Lionel Dupret (APGN), Thierry Rebours (AGPAH), Isabelle Aubron (PNR Normandie Maine) Milieu terrestre : Sylvain Diquélou (Université de Caen), Catherine Zambettakis (CBNB), Claire Mouquet, Antoine Racine et Emmanuel Jacob (GRETIA) Milieu marin : Olivier Timsit et Alexandrine Baffreau (GEMEL-N) Illustrations du document : A.-L. Giommi, sauf mention particulière Référence du document : GIOMMI A.-L., 2020 – Plan de gestion 2020-2024 de la réserve naturelle nationale Falaise du Cap Romain, section A (diagnostic). Département du Calvados, 101 p. + annexes 2 Plan de gestion 2020-2024 de la RNN Falaise du Cap Romain 29 janvier 2021 Sommaire Sommaire ........................................................................................................................................................................... 3 Préambule ......................................................................................................................................................................... -

210X297 Fichescalvadosavelo2021 2.Indd
Véloroute Retrouvez la carte complète du pays d’Auge des itinéraires dans le document Section de Cabourg à Mézidon-Canon Le Calvados à Vélo 2021 D513 Houlgate Auberville Cabourg Baie Merville- Franceville- 3 km 7 D514 2 de l’Orne Le Hôme D D Plage 2 1 4 6 Varaville 1 3 Ouistreham D DSaint-Vaast-2 4en-Auge D35A Gonneville- 1 4 Dives- D5 D 4 sur-Mer 1 Cabourg 8 3 sur-Mer 4 51 D 2 D D 45 A Heuland Saint-Aubin- D400A d'Arquenay Sallenelles 23 2 Branville D Grangues Gonneville- D49 B 4 45 1 0 D 0 Dives-sur- 5 4 en-Auge D 4 D Mer 27 Périers-en- Douville- D D 9 D en-Auge 5 Auge 4 A 5 D400A 6 13 Varaville C 7 7 2 Amfreville D5 D2 s D Danestal 5 e D 9 v 2 D i Brucourt 36 10 km Angerville A Petiville D 13 Pegasus Bréville- a re Bridge L Cricqueville- nc les-Monts L'A D400 en-Auge D Bavent Cresseveuille 2 7 Ranville D in 6 37 D2 rs 2 24 D D u D 0 224 Photo © Calvados Attractivité 4 29 Dozulé 28 D 2 O 5 7 3 67 Hérouvillette 6 D l a D Hameau n Saint-Léger- 3 a 3 7 de Bricqueville C Sites propres 2 Dubosq 2 Beaufour- D Putot- Sites propres Escoville Bois de Goustranville Druval Voies partagées 9 en-Auge D Voies partagées 5 km 4 SitesItinéraires propres en cours 2 D Bavent Saint-Jouin 7 D 6 VoiesItinérairesd’aménagement partagées en cours 3 D228 7 3 D675 l C 1 a d’aménagement A n BoucleItinéraires vélo en cours a Bures sur C Boucled’aménagement vélo A D 4 Basseneville 8 SERVICES Dives 2 5 2 Boucle vélo Cuverville Touréville D d 46 SERVICESOffice de Tourisme n D1 a OfficeSyndicat de d’initiativeTourisme Saint- r 6A SERVICES 2,2 km G D14 Giberville D22 -

Recueil Des Actes Administratifs Spécial N°14-2020-094
RECUEIL DES ACTES ADMINISTRATIFS SPÉCIAL N°14-2020-094 CALVADOS PUBLIÉ LE 17 JUILLET 2020 1 Sommaire Direction départementale de la cohésion sociale 14-2020-06-13-001 - Liste des admis au BNSSA (1 page) Page 3 Direction départementale des territoires et de la mer du Calvados 14-2020-07-17-001 - Arrêté préfectoral portant agrément de la Société des Eaux de Trouville Deauville et Normandie pour la réalisation des opérations de vidange, transport et élimination des matières extraites des installations d'assainissement non collectif (4 pages) Page 5 14-2020-07-16-005 - Arrêté préfectoral prescrivant la restauration de la continuité écologique au point de diffluence de la rivière Orbiquet et du ruisseau Graindin et sur la rivière Orbiquet au droit du vannage du Carmel, commune de LISIEUX (5 pages) Page 10 Préfecture du Calvados 14-2020-07-17-002 - 20200717-ArrêtéGrandsElecteurs (1 page) Page 16 14-2020-07-17-003 - 20200717-GRANDS ELECTEURS (48 pages) Page 18 14-2020-07-17-004 - Arrêté préfectoral du 17 juillet 2020 portant réglementation de la circulation sur les autoroutes A13 et A132 (4 pages) Page 67 2 Direction départementale de la cohésion sociale 14-2020-06-13-001 Liste des admis au BNSSA Jury du 13 juin 2020 Direction départementale de la cohésion sociale - 14-2020-06-13-001 - Liste des admis au BNSSA 3 Direction départementale de la cohésion sociale - 14-2020-06-13-001 - Liste des admis au BNSSA 4 Direction départementale des territoires et de la mer du Calvados 14-2020-07-17-001 Arrêté préfectoral portant agrément de la Société des -

Plan De Ville
TOURISME 181140_CABOURG_OT_95x36.qxp_Mise en page 1 27/11/2018 16:34 Page 1 TOURISME TOURISME TOURISME TOURISME A B C D E PISCINE F G H n Hôtel les bains de Cabourg**** ÉCOLE CLUB DE PLAGE DE VOILE n ÉTABLISSEMENT CLUB DE PLAGE Restaurant DES BAINS ÉCOLE n 5 salles de réunion DESCENTE BATEAUX CLUB DE PLAGE DE CHAR À VOILE DESCENTE BATEAUX n Centre de thalassothérapie & Spa] [ POSTE DE POSTE DE POSTE DE GOLF MINIATURE POSTE DE ÉCOLE DE TOURISME PLAGE KITE SURF 02 50 22 1000 / www.thalazur.fr/hotel-cabourg / [email protected] SECOURS 1 SECOURS 2 SECOURS 4 (CENTRAL) SECOURS 5 E F PROMENADE MARCEL PROUST PROMENADE MARCEL PROUST GRAND HÔTEL Jardins de l’Hôtel de Ville RÉS. LA RÉS. CABOURG 2000 1 RÉS. CAP CABOURG DUNES G POINTE DE PLATANES DES TAMARIS DES AV. MERMOZ AV. 14390 CABOURG AV. DU MARÉCHAL FOCH AV. DU SYCOMORES COMMANDANT TOUCHARD CABOURG BIZONTINE VILLAGE THÉÂTRE MARIN AV. DES VOILIERS MORIMBAU AV. CHEMINS PÉDESTRES DES T: +33 (0)2 31 06 20 00 DE VACANCES BAINS DES COMMUNE SERGENT AV. DES SAPINS C AV. DU RUE G. AFFRE BRIAND JOFFRE AV. DURAND DES AULNAIES DES PASSERELLE www.cabourg-tourisme.fr SWEET HOME ARISTIDE AV. DU AV. MARÉCHAL AV. AV. GOLF MAINE SPORTING PEUPLIERS JET SKI AV. DES AV. DES ALGUES MARINES 2 D AV. DES VALÉES [email protected] AV. 18 TROUS DU HÔME-VARAVILLE RÉS. CLUB AV. A. PREMPAIN PL. MARCEL PROUST AV. DU ROI ALBERT 1ER JARDINS DU CASINO MERMOZ THALASSOTHÉRAPIE HORTENSIA TENNIS AV. GEORGES CLÉMENCEAU DIDIER AL. -

20Caen Cabourg Deauville
LIGNE CAEN CABOURG 20 DEAUVILLE HORAIRES VALABLES DU 02 SEPTEMBRE 2021 AU 06 JUILLET 2022 INCLUS* *Horaires susceptibles d’être modifiés au 19 décembre 2021. Car en coordination avec les trains lun au ven en provenance de PARIS sam et dim lun au ven Autres correspondances NOMAD sam et dim Lundi Lundi Lundi Lundi, Lundi Horaires Deauville Lundi Lundi Lundi Lundi Lundi Dimanches et fêtes Lundi au Samedi au au Lundi au Vendredi au au au au Samedi Lundi au vendredi Mardi, au au au vendredi samedi samedi samedi vendredi Jeudi, vendredi (sauf le 1er mai) < > le Havre en page 2 samedi (3) (2) vendredi samedi (1) Vendredi samedi (1) samedi Période de validité TA TA TA TA TA TA TA PS TA TA TA TA TA PS PVS TA PS TA TA TA TA TA TA TA TA TA TA CAEN / Gare Routière 07:30 07:30 09:13 10:25 11:25 12:30 13:26 13:30 14:30 15:20 17:03 16:53 17:30 17:20 18:08 18:08 18:41 19:30 20:15 07:55 09:25 11:25 14:25 15:30 18:30 20:20 CAEN / Demi-Lune 07:33 07:33 09:16 10:28 11:28 12:33 13:29 13:33 14:33 15:23 17:06 16:56 II 18:11 18:11 18:44 19:33 20:18 07:58 09:28 11:28 14:28 15:33 18:33 20:23 MONDEVILLE / Maison du Peuple 07:34 07:34 09:17 10:29 11:29 12:34 13:30 13:34 14:34 15:24 17:07 16:57 II 18:12 18:12 18:45 19:34 20:19 07:59 09:29 11:29 14:29 15:34 18:34 20:24 MONDEVILLE / Route de Cabourg 07:35 07:35 09:18 10:30 11:30 12:35 13:31 13:35 14:35 15:25 17:08 16:58 II 18:13 18:13 18:46 19:35 20:20 08:00 09:30 11:30 14:30 15:35 18:35 20:25 MONDEVILLE / Grands Bureaux 07:37 07:37 09:20 10:32 11:32 12:37 13:33 13:37 14:37 15:27 17:10 17:00 17:37 17:27 18:15 18:15 -

20Le Havre Deauville Caen
LIGNE LE HAVRE DEAUVILLE CAEN 20 ER HORAIRES VALABLES DU 7 JUILLET 2021 AU 1 SEPTEMBRE 2021 INCLUS lun au ven Autres correspondances NOMAD sam Lundi Lundi Navette Honfleur - Deauville en page 3 au Lundi au Lundi au samedi vendredi au samedi vendredi LE HAVRE / Gare Routière 09:00 18:05 20:15 HONFLEUR* / Village des Marques 09:21 I I LA RIVIÈRE ST-SAUVEUR* / Rue du Bourg I 11:21 18:28 20:38 LA RIVIÈRE ST-SAUVEUR* / Saint-Clair I 11:23 18:30 20:40 HONFLEUR* / Gare Routière (Arrivée) 09:26 11:28 18:34 20:45 HONFLEUR* / Gare Routière (Départ) 06:25 08:00 08:40 09:28 11:30 13:20 15:30 16:00 19:01 19:30 I HONFLEUR* / Les Marronniers 06:27 08:03 08:43 I 11:33 13:23 15:33 16:03 19:04 19:33 20:43 ST-GATIEN-DES-BOIS* / Église 06:38 08:14 08:54 I 11:44 13:34 15:44 16:14 19:15 19:44 ST-GATIEN-DES-BOIS* / Aéroport 06:42 08:18 08:58 I 11:48 13:38 15:48 16:18 19:19 19:48 CRICQUEBŒUF* / Pôle de Santé I I 09:02 I 11:52 13:42 I 16:22 19:23 I TOUQUES* / La Croix Sonnet 06:46 08:22 09:04 I 11:54 13:44 15:52 16:24 19:25 19:52 TOUQUES* / Église 06:50 08:26 09:08 I 11:59 13:49 15:57 16:28 19:29 19:56 TOUQUES* / Reine Mathilde 06:51 08:27 09:09 I 12:00 13:50 15:58 16:29 19:30 19:57 DEAUVILLE* / Gare Routière (Arrivée) 06:57 08:33 09:15 09:59 12:09 13:57 16:07 16:35 19:36 20:03 DEAUVILLE* / Gare Routière (Départ) 07:05 07:30 08:35 09:20 10:10 11:15 11:45 12:15 13:00 14:15 15:05 16:10 16:40 17:40 18:10 18:40 19:40 20:35 21:35 DEAUVILLE* / Art Station 07:07 07:32 08:38 09:23 10:13 11:18 I 12:18 13:03 14:19 15:08 16:14 16:43 I 18:13 18:43 19:42 20:37 21:37 DEAUVILLE* -

Les Contacts
Les contacts du festival 3 > 18 JUIN 2016 AUTHIE ........................................ 02 31 71 11 00 BAVENT ....................................... 02 31 78 88 25 BRETTEVILLE-SUR-LAIZE. 02 31 08 49 51 BRETTEVILLE-SUR-ODON ..................... 02 31 73 98 91 CAEN .......................................... 02 31 30 47 00 CAIRON ....................................... 02 31 80 99 03 CORMELLES-LE-ROYAL ........................ 02 31 52 18 54 ÉVRECY ....................................... 02 31 80 57 49 FONTAINE-ÉTOUPEFOUR ...................... 02 31 26 30 32 ISIGNY-SUR-MER .............................. 02 31 10 66 16 LISIEUX Médiathèque ................................ 02 31 48 41 00 Tanit théâtre ................................ 02 31 62 66 08 LIVAROT-PAYS D’AUGE ........................ 02 31 61 88 18 MERVILLE-FRANCEVILLE-PLAGE .............. 09 67 02 27 70 MOUEN ........................................ 07 86 65 40 57 ORBEC ........................................ 06 03 54 75 85 OUILLY-LE-TESSON ............................ 02 31 90 96 02 PAYS DE FALAISE ............................. 02 31 41 65 45 10e ÉDITION RANVILLE ..................................... 02 31 78 18 63 ROCQUANCOURT .............................. 02 31 79 86 25 SOULEUVRE-EN-BOCAGE. 02 31 66 94 64 THAON ........................................ 02 31 08 16 35 VALDALLIÈRE ................................. 02 31 09 09 18 conception : www.pimenta-studio.fr BIBLIOTHÈQUE DU CALVADOS 02 31 78 78 87 bdp.calvados.fr Président du Département du Calvados So14, tellement Calvados -

Upper Triassic Corals and Carbonate Reef Facies from the Martin Bridge and Hurwal Formations, Wallowa Terrane (Oregon)
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 2010 UPPER TRIASSIC CORALS AND CARBONATE REEF FACIES FROM THE MARTIN BRIDGE AND HURWAL FORMATIONS, WALLOWA TERRANE (OREGON) Megan Ruth Rosenblatt The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Rosenblatt, Megan Ruth, "UPPER TRIASSIC CORALS AND CARBONATE REEF FACIES FROM THE MARTIN BRIDGE AND HURWAL FORMATIONS, WALLOWA TERRANE (OREGON)" (2010). Graduate Student Theses, Dissertations, & Professional Papers. 1354. https://scholarworks.umt.edu/etd/1354 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. UPPER TRIASSIC CORALS AND CARBONATE REEF FACIES FROM THE MARTIN BRIDGE AND HURWAL FORMATIONS, WALLOWA TERRANE (OREGON) By MEGAN RUTH ROSENBLATT Bachelor of Science, College of Charleston, Charleston SC, 2006 Thesis Presented in partial fulfillment of the requirements for the degree of Master of Science in Geosciences The University of Montana Missoula, MT December 2010 Approved by: Perry Brown, Associate Provost for Graduate Education Graduate School George Stanley, Ph.D., Chair Geosciences Marc Hendrix, Ph.D. Geosciences Jon Graham, Ph.D. Mathematical Sciences i ACKNOWLEDGEMENTS I would like to thank the National Science Foundation for field and laboratory funding from a grant awarded to George Stanley. For tuition and salary as a Research Assistant I would like to thank the Innovative Technology Experiences for Students and Teachers (ITEST) section of the National Science Foundation; a grant awarded to Heather Almquist and George Stanley. -

Scleractinia from the Upper Portlandian of Tisbury, Wiltshire, England
ACT A PAL A EON T 0 LOG ICA POLONICA Vol. XV 1970 No.4 EWA RONIEWICZ SCLERACTINIA FROM THE UPPER PORTLANDIAN OF TISBURY, WILTSHIRE, ENGLAND Abstract. - Four species of Scleractinia: Pseudodiplocoenia oblonga (Fleming), Ellipsasteria gracilis n. gen., n. sp., Edwardsastraea tisburiensis n. gen., n. sp. and Ebrayia dightonthomasi n. sp., from the uppermost Portlandian of Tisbury, west of Salisbury, Wiltshire, England, are here described. The histological structure of the skeleton of Pseudodiplocoenia oblonga, preserved in silicified colonies is presented. INTRODUCTION A little known coral fauna occurs in the uppermost beds of the Upper Portlandian near Tisbury (West of Salisbury, Wiltshire, England). The preservation of colonies in a completely silicified. state is a peculiarity of this fauna which, containing representatives of the Recent families, Helia straeidae and Faviidae, deviates in character from typical Jurassic faunas. Only one of the species which occur at this locality has so far been described by H. Milne-Edwards &J. Haime (1851) in Part II of "A Mono graph of the British Fossil Corals" as Isastraea oblonga (Fleming). This name was subsequently used to designate all corals from Tisbury in the collections of the British Museum and the Geological Survey Museum, including the representatives of the three new species described here. A fairly considerable specific differentiation of the collection under study, which is an accidental collection, suggests that the fauna of corals from Tisbury might be much richer. As a result of a complete silification of the colonies, macroscopic cha racters of the skeleton are clearly visible. In addition, the histological structure of the skeleton is preserved in specimens of P.