Sicily Channel/Tunisian Plateau: Status of Cetaceans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Improved Modelling of the Messinian Salinity Crisis and Conceptual Implications
Palaeogeography, Palaeoclimatology, Palaeoecology 238 (2006) 349–372 www.elsevier.com/locate/palaeo Improved modelling of the Messinian Salinity Crisis and conceptual implications Paul-Louis Blanc Institut de Radioprotection et de Sûreté Nucléaire, Boîte Postale 17, 92262 Fontenay-aux-roses Cedex, France Received 17 April 2003; accepted 7 March 2006 Abstract This paper presents the last developments of a simple oceanographic modelling of the water and salt budgets of the Mediterranean Sea during the late Miocene Salinity Crisis. The Messinian Mediterranean is treated as analogous to the present one, i.e. divided into two main basins separated by a sill at shallow or intermediate depth. When the supply of marine water from the Atlantic is progressively reduced, both basins undergo a rise in salinity, until saturation is reached: this is when the true evaporitic sedimentation begins, before the level drawdown. The partition of the Mediterranean into two mains basins causes a shift in the evaporitic sedimentation from the distal (eastern) basin to the proximal (western) basin, so that the evaporitic deposits are not fully contemporaneous in the western and the eastern basin. The drawdown is limited by the equilibrium of the evaporation and chemical activity of the brines at the surface, against the surface area and evaporation. Attempts at adjusting the model both to an accurate stratigraphic frame and to a rough budget of the evaporites shows that the Upper Evaporites and brackish-water Lago-mare series must have been deposited as secondary deposits, after the closure of the Atlantic passages was completed. The present evaporitic potentialities of the Mediterranean Sea remains quite as strong as during the Miocene, so that climatic change cannot be inferred from the MSC itself. -

Marine Mammals and Sea Turtles of the Mediterranean and Black Seas
Marine mammals and sea turtles of the Mediterranean and Black Seas MEDITERRANEAN AND BLACK SEA BASINS Main seas, straits and gulfs in the Mediterranean and Black Sea basins, together with locations mentioned in the text for the distribution of marine mammals and sea turtles Ukraine Russia SEA OF AZOV Kerch Strait Crimea Romania Georgia Slovenia France Croatia BLACK SEA Bosnia & Herzegovina Bulgaria Monaco Bosphorus LIGURIAN SEA Montenegro Strait Pelagos Sanctuary Gulf of Italy Lion ADRIATIC SEA Albania Corsica Drini Bay Spain Dardanelles Strait Greece BALEARIC SEA Turkey Sardinia Algerian- TYRRHENIAN SEA AEGEAN SEA Balearic Islands Provençal IONIAN SEA Syria Basin Strait of Sicily Cyprus Strait of Sicily Gibraltar ALBORAN SEA Hellenic Trench Lebanon Tunisia Malta LEVANTINE SEA Israel Algeria West Morocco Bank Tunisian Plateau/Gulf of SirteMEDITERRANEAN SEA Gaza Strip Jordan Suez Canal Egypt Gulf of Sirte Libya RED SEA Marine mammals and sea turtles of the Mediterranean and Black Seas Compiled by María del Mar Otero and Michela Conigliaro The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN. Published by Compiled by María del Mar Otero IUCN Centre for Mediterranean Cooperation, Spain © IUCN, Gland, Switzerland, and Malaga, Spain Michela Conigliaro IUCN Centre for Mediterranean Cooperation, Spain Copyright © 2012 International Union for Conservation of Nature and Natural Resources With the support of Catherine Numa IUCN Centre for Mediterranean Cooperation, Spain Annabelle Cuttelod IUCN Species Programme, United Kingdom Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the sources are fully acknowledged. -

Reef Building Mediterranean Vermetid Gastropods: Disentangling the Dendropoma Petraeum Species Complex J
Research Article Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available on line at http://www.medit-mar-sc.net DOI: http://dx.doi.org/10.12681/mms.1333 Zoobank: http://zoobank.org/25FF6F44-EC43-4386-A149-621BA494DBB2 Reef building Mediterranean vermetid gastropods: disentangling the Dendropoma petraeum species complex J. TEMPLADO1, A. RICHTER2 and M. CALVO1 1 Museo Nacional de Ciencias Naturales (CSIC), José Gutiérrez Abascal 2, 28006 Madrid, Spain 2 Oviedo University, Faculty of Biology, Dep. Biology of Organisms and Systems (Zoology), Catedrático Rodrigo Uría s/n, 33071 Oviedo, Spain Corresponding author: [email protected] Handling Editor: Marco Oliverio Received: 21 April 2014; Accepted: 3 July 2015; Published on line: 20 January 2016 Abstract A previous molecular study has revealed that the Mediterranean reef-building vermetid gastropod Dendropoma petraeum comprises a complex of at least four cryptic species with non-overlapping ranges. Once specific genetic differences were de- tected, ‘a posteriori’ searching for phenotypic characters has been undertaken to differentiate cryptic species and to formally describe and name them. The name D. petraeum (Monterosato, 1884) should be restricted to the species of this complex dis- tributed around the central Mediterranean (type locality in Sicily). In the present work this taxon is redescribed under the oldest valid name D. cristatum (Biondi, 1857), and a new species belonging to this complex is described, distributed in the western Mediterranean. These descriptions are based on a comparative study focusing on the protoconch, teleoconch, and external and internal anatomy. Morphologically, the two species can be only distinguished on the basis of non-easily visible anatomical features, and by differences in protoconch size and sculpture. -

Finding of Uncommon Cephalopods
Cah. Biol. Mar. (1994),35: 339-345 Roscoff Finding of uncommon cephalopods (Ancistroteuthis lichtensteinii, Histioteuthis bonnellii, Histioteuthis reversa) and first record of Chiroteuthis veranyi in the Ionian Sea. A. Tursi, G. D'Onghia, A. Matarrese, P. Panetta, P. Maiorano Institute of Zoology and Comparative Anatomy - University - Via Orabona, 4 - 70125 Bari (ltaly) Abstract : Finding of some uncommon cephalopods. AnCÎslrolelilhis lichtensteinii, Histiotellthis bonne/Iii, H. rCl'ersa and the first record of Chirotellthis Feranyi in the Ionian Sea are reported here. Data were collected during a trawl survey carried out on red shrimp grounds during August 1993. Résumé : Dans ce travail, les auteurs analysent des espèces de Céphalopodes peu communes ou rares en Méditerranée, Ancislrotellthis Iichlensteinii, Histiotellthis bonnellii, H. reversa et ils signalent pour la première t"ois la présence de Chirotellthis l'eranyi en mer Ionienne. Les exemplaires ont été capturés au cours d'une campagne de chalutage expérimentale, sur des fonds ù crevettes rouges, au mois d'Août 1993. INTRODUCTION The SCal"CÎty of ecological knowledge on many species of cephalopods is certainly due to the technical problems of field observation and sampling. Information on distribution and abundance come mainly from CUITent research of biologi cal oceanography, fishing activities, analysis on stomach contents of mmine mammals and pelagic fishes. Recently, observations from submersibles have provided very interesting data about several aspects of the biology and ecology of cephalopods (Vecchione & Roper, 1991). Cephalopods are captured commercialy and for research purposes using a valiety of gears, primarily jigging machines, trawls and drift nets (Roper, 1991). While jigging, pela gic seining and potting are less destructive techniques that target specific sizes and species, trawling and drift netting are non-selective methods as far as size and specieS' are concer ned. -

Cephalopoda: Histioteuthidae) in the Southern Tyrrhenian Sea (Western Mediterranean)
NOT TO BE CITED WITHOUT PRIOR REFERENCE TO THE AUTHOR(S) Northwest Atlantic Fisheries Organization Serial No. N4560 NAFO SCR Doc. 01/165 SCIENTIFIC COUNCIL MEETING - SEPTEMBER 2001 (Deep-sea Fisheries Symposium – Poster) Occurrence of Histioteuthis bonnellii and Histioteuthis reversa (Cephalopoda: Histioteuthidae) in the Southern Tyrrhenian Sea (Western Mediterranean) by D. Giordano*, G. Florio*, T. Bottari*, and S. Greco** *Istituto Sperimentale Talassografico C.N.R., Spianata S. Raineri, 86 98100 Messina, Italy ** ICRAM, Istituto per la Ricerca Scientifica Applicata al Mare, Via dei Casalotti, 300 00166 Roma -Italy Abstract Data on occurrence of Histioteuthis bonnellii and Histioteuthis reversa collected in the southern Tyrrhenian Sea from 1994 to 1998 during five trawl surveys of the Medits eight trawl survey of the Grund project are reported. The International bottom trawl survey (the MEDITS programme) has been designed from a European Commission’s initiative to produce biological data on the demersal resources along the coasts of the four Mediterranean countries of the European Union (Spain, France, Italy and Greece). The main objective was to obtain independent knowledge useful for the fishery management, in an area where it is difficult to follow in detail the exploitation patterns of the fishing fleets. In Italian seas, before 1985, there was no research at national level on biological aspect and assessment of demersal resources. Within the framework of the first national plan (1985-1988) of the Law 41/82, three main research groups to assess demersal resources were organized: Tyrrhenyan group, Adriatic and Ionian group and Sicily group. All the seas were covered, with the exception of Ionian part of Sicily. -

Western Ligurian Sea and Genoa Canyon Important Marine Mammal Area – IMMA
Western Ligurian Sea and Genoa Canyon Important Marine Mammal Area – IMMA Description Cuvier’s beaked whale ( Ziphius cavirostris G. Area Size Cuvier, 1823), is the only beaked whale 8,526 km 2 regularly inhabiting the Mediterranean Sea area, where this species has been found Qualifying Species and Criteria associated with continental slope and with Cuvier's beaked whale - submarine canyons and seamounts areas. The Ziphius cavirostris Cuvier's beaked whale Mediterranean Criterion B (i, ii); C (i, ii) subpopulation was being re-assessed in early Marine Mammal Diversity 2017 with the expectation that it would meet Criterion D (ii) at least one of the Red List criteria for [Stenella coeruleoalba, Physeter Vulnerable based on the results of basin-wide macrocephalus, Globicephala melas, density surface modelling (Cañadas et al Balaenoptera physalus, Grampus griseus ] 2016). Cuvier’s beaked whales have been Summary sighted in the Ligurian Sea especially in waters over and around canyons (Azzellino et al. The Genoa Canyon, located in the 2008, 2011, 2012; Azzellino et al. In press; westernmost part of the Ligurian Sea, has been identified as a high-density area for a Azzellino & Lanfredi, 2015; D’Amico et al., resident population of Mediterranean 2003; Lanfredi et al., 2016). In particular, the Cuvier’s beaked whales ( Ziphius cavirostris ). Genoa Canyon area has been identified as a A high correlation was also observed high-density area for Cuvier’s beaked whales between the presence of Cuvier’s beaked (MacLeod and Mitchell, 2006; Moulins et al., whales and the underlying canyon area; this 2007; Tepsich et al., 2014, Cañadas et al., has been validated by modelling studies. -

Marine Geophysical Research
Marine Geophysical Research Seabed mapping in the Pelagie Islands Marine Protected Area (Sicily Channel, southern Mediterranean) using Remote Sensing Object Based Image Analysis (RSOBIA) --Manuscript Draft-- Manuscript Number: MARI-D-18-00014R2 Full Title: Seabed mapping in the Pelagie Islands Marine Protected Area (Sicily Channel, southern Mediterranean) using Remote Sensing Object Based Image Analysis (RSOBIA) Article Type: Original Research Keywords: Multibeam bathymetry; backscatter; benthoscapes; seabed classification; ground- truth data; Posidonia oceanica; coralligenous habitat. Corresponding Author: Sara Innangi Istituto per l'ambiente marino costiero Consiglio Nazionale delle Ricerche ITALY Corresponding Author Secondary Information: Corresponding Author's Institution: Istituto per l'ambiente marino costiero Consiglio Nazionale delle Ricerche Corresponding Author's Secondary Institution: First Author: Sara Innangi First Author Secondary Information: Order of Authors: Sara Innangi Renato Tonielli Claudia Romagnoli Francesca Budillon Gabriella Di Martino Michele Innangi Roberta La Terza Tim Le Bas Claudio Lo Iacono Order of Authors Secondary Information: Funding Information: Abstract: AcceptedIn this paper we present the seabed maps of the shallow-water areas of Lampedusa and Linosa, belonging to the Pelagie Islands Marine Protected Area. Two surveys were carried out (“Lampedusa2015” and “Linosa2016”) to collect bathymetric and acoustic backscatter data through the use of a Reson SeaBat 7125 high-resolution multibeam system. Ground-truth -

Marine Turtles
UNEP/MED IG.24/22 Page 372 Decision IG.24/7 Strategies and Action Plans under the Protocol concerning Specially Protected Areas and Biological Diversity in the Mediterranean, including the SAP BIO, the Strategy on Monk Seal, and the Action Plans concerning Marine Turtles, Cartilaginous Fishes and Marine Vegetation; Classification of Benthic Marine Habitat Types for the Mediterranean Region, and Reference List of Marine and Coastal Habitat Types in the Mediterranean The Contracting Parties to the Convention for the Protection of the Marine Environment and the Coastal Region of the Mediterranean and its Protocols at their 21st Meeting, Recalling the outcome document of the United Nations Conference on Sustainable Development, entitled “The future we want”, endorsed by the General Assembly in its resolution 66/288 of 27 July 2012, in particular those paragraphs relevant to biodiversity, Recalling also General Assembly resolution 70/1 of 25 September 2015, entitled “Transforming our world: the 2030 Agenda for Sustainable Development”, and acknowledging the importance of conservation, the sustainable use and management of biodiversity in achieving the Sustainable Development Goals, Recalling further the United Nations Environment Assembly resolutions UNEP/EA.4/Res.10 of 15 March 2019, entitled “Innovation on biodiversity and land degradation”, Bearing in mind the international community’s commitment expressed in the Ministerial Declaration of the United Nations Environment Assembly at its fourth session to implement sustainable ecosystems -
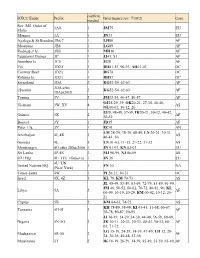
DXCC Entity Prefix Confirm. Needed Grid Square.Rev: 7/30/21 Cont. Sov
confirm. DXCC Entity Prefix Grid Square.rev: 7/30/21 Cont. needed Sov. Mil. Order of 1A0 1 JM75 EU Malta Monaco 3A 1 JN33 EU Agalega & St.Brandon 3B6,7 1 LH89 AF Mauritius 3B8 1 LG89 AF Rodriguez Is. 3B9 1 MH10 AF Equatorial Guinea 3C 1 JJ41, 51 AF Annobon Is. 3C0 1 JI28 AF Fiji 3D2/f 3 RH81-83, 90-93, AH01-03 OC Conway Reef 3D2/c 1 RG78 OC Rotuma Is. 3D2/r 1 RH87 OC Swaziland 3DA 2 KG52-54, 62-63 AF 3DA after eSwatini 2 KG52-54, 62-63 AF 18Apr2018 Tunisia 3V 2 JM33-34, 40-47, 50-57 AF OJ28-29, 39, OK20-21, 27-38, 40-46, Vietnam 3W, XV 4 AS OL00-01, 10-12, 20 IJ39, 48-49, 57-59, IK20-21, 30-32, 40-42, Guinea 3X 2 AF 50-52 Bouvet 3Y 1 JD15 AF Peter 1 Is. 3Y 1 EC41 AN LM 28-29, 38-39, 48-49, LN 20-21, 30-31. Azerbaijan 4J, 4K 3 AS 40-41, 50 Georgia 4L 3 LN 01-03, 11-13, 21-22, 31-32 AS Montenegro 4O after 28Jun2006 1 JN 91-93, KN 02-03 EU Sri Lanka 4P-4S 2 MJ 96-99, NJ 06-09 AS ITU HQ 4U_ITU (Geneva) 1 JN 36 EU 4U_UN United Nations HQ 1 FN 30 NA (New York) Timor-Leste 4W 1 PI 20-21, 30-31 OC Israel 4X, 4Z 3 KL 79, KM 70-73 AS JL 45-49, 53-59, 63-69, 72-79, 83-89, 91-99, JM 40, 50-52, 60-62, 70-72, 80-81, 90, KL Libya 5A 2 AF 00-09, 10-19, 20-29, KM 00-02, 10-12, 20- 21 Cyprus 5B 2 KM 64-65, 74-75 AS KH 78-89, 98-99, KI 43-45, 51-58, 60-67, Tanzania 5H-5I 3 AF 70-78, 80-87, 90-95 JJ 16-19, 24-29, 34-39, 44-49, 56-59, 68-69, Nigeria 5N-5O 3 JK 10-11, 20-23, 30-33, 40-43, 50-53, 60- AF 63, 71-72 LG 15-19, 24-29, 34-39, 47-49, LH 12, 20- Madagascar 5R-5S 2 AF 24, 30-36, 40-48, 53-56 Mauritania 5T 2 IK 16-19, 26-29, 34-39, 45-49, 55-59, 65-69, -

Modelling Global-Scale Climate Impacts of the Late Miocene Messinian Salinity Crisis
This is a repository copy of Modelling global-scale climate impacts of the late Miocene Messinian Salinity Crisis. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/80166/ Version: Published Version Article: Ivanovic, RF, Valdes, PJ, Flecker, R et al. (1 more author) (2014) Modelling global-scale climate impacts of the late Miocene Messinian Salinity Crisis. Climate of the Past, 10 (2). 607 - 622. ISSN 1814-9324 https://doi.org/10.5194/cp-10-607-2014 Reuse Unless indicated otherwise, fulltext items are protected by copyright with all rights reserved. The copyright exception in section 29 of the Copyright, Designs and Patents Act 1988 allows the making of a single copy solely for the purpose of non-commercial research or private study within the limits of fair dealing. The publisher or other rights-holder may allow further reproduction and re-use of this version - refer to the White Rose Research Online record for this item. Where records identify the publisher as the copyright holder, users can verify any specific terms of use on the publisher’s website. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Clim. Past, 10, 607–622, 2014 Open Access www.clim-past.net/10/607/2014/ Climate doi:10.5194/cp-10-607-2014 © Author(s) 2014. CC Attribution 3.0 License. -

Navionics.Com AMERICAS
Large Charts AFRICA CODE TITLE COVERAGE DESCRIPTION AF036L SOUTH WEST AFRICA South Gabon, Angola, South Africa, Namibia, Tristan da Cunha, Gough Islands and Prince Edward Islands. AF037L AFRICA SE/MADAGASCAR From Durban to Mchinga Bay, Tromelin Island, Mozambique Channel, Madagascar, Comores, Mauritius, La Reunion, Cargados carajos. AF038L AFRICA MIDDLE EAST Mocambique to Lamu Bay, from Nosy Lava, Antsiranana to Sambava in Madagascar, Comores, Seychelles, Farquhar Islands. AF039L AFRICA NE Somalia to Sadani in Tanzania, Northern Zanzibar Island, Pemba Island, Suqutra, Seychelles Islands. AT167L SAHARA W./GUINEA G. Western Sahara, Mauritania, Senegal, Guinea-Bissau, Guinea, Sierra Leone, Liberia, Cote D’Ivoire, Ghana, Benin, Nigeria, Cameroon, Gabon and Cape Verde Islands. ME018L RED SEA Red Sea ME019L GULF OF ADEN From Dolphin Cove in Eritrea to Dante in Somalia, Socotra, from Jahfuf Bay in Saudi Arabia to Sadh in Oman. ME020L GULF OF OMAN From Nay Band to Chah Bahar in Iran. From Sir Bani Yas in United Arab Emirates to Sadh in Oman. ME021L WESTERN PERSIAN GULF From Sir Bani Yas in United Arab Emirates, Qatar, Saudi Arabia, Kuwait and Abadan to Nay Band in Iran. AMERICAS CODE TITLE COVERAGE DESCRIPTION CX141L NORTH CUBA North Cuba from Bahiade Cochinos to Cape San Antonio to Santiago de Cuba, including Cay Sal Bank. CX142L SOUTH CUBA-JAMAICA Entire Cayman Islands, Jamaica and South Cuba from La Fe to Bahia de Banes, including Island de la juventud. CX143L HAITI-DOMINICAN REP. Entire Island of Haiti, Dominican Republic and East Cuba from Ensenada Sabanalamar to Baracoa, West Puerto Rico from Bahia de Guanica to Bahia de Aguadilla, including Winward Passage, Mona Passage, Isla de Mona. -

In the Mediterranean Surface and Deep Waters
E3S Web of Conferences 1, 17008 (2013) DOI: 10.1051/e3sconf/20130117008 C Owned by the authors, published by EDP Sciences, 2013 Dissolved gaseous Hg (DGM) in the Mediterranean surface and deep waters J. Kotnik1 and M. Horvat1 1 Jozef Stefan Institute, Department of Environmental Sciences, Jamova 39, Ljubljana, Slovenia, [email protected] Abstract. Dissolved gaseous mercury (DGM) was studied in surface and deep waters of the Mediterranean Sea for last 12 years during several oceanographic cruises on board the Italian research vessel Urania and covered both Western and Eastern Mediterranean Basins as well as Adriatic Sea. DGM was measured together with other mercury species (RHg - reactive Hg, THg - total Hg, MeHg - monomethyl Hg and DMeHg - dimethylmercury), and with some water quality parameters in coastal and open sea deep water profiles, however only DGM will be discussed here. DGM represents a considerable portion of THg (average of about 20 %) in Mediterranean waters. Spatial and seasonal variations of measured DGM concentrations were observed in different indentified water masses as well as iwere observed. DGM was the highest in the northern Adriatic, most polluted part of the Mediterranean Sea as the consequence of Hg mining in Idrija and heavy industry of northern Italy.Generally, average DGM concentration was higher in W and E Mediteranean Deep Waters (WMDW and EMDW) and Leavantine Intermediate Water (LIW) than overlaying Modified Atlantic Water (MAW), however it was the highest in N Adriatic Surface waters and consequently in out flowing Adriatic Deep Waters (ADW). In deep water profiles the portion of DGM typically increased at depths with oxygen minimum and then towards the bottom, especially in areas with strong tectonic activity (Alboran Sea, Strait of Sicily, Tyrrhenian Sea), indicating its bacterial and/or geotectonic origin.