Pesticide Risk Assessment (S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

Common and Chemical Names of Herbicides Approved by the WSSA
Weed Science 2010 58:511–518 Common and Chemical Names of Herbicides Approved by the Weed Science Society of America Below is the complete list of all common and chemical of herbicides as approved by the International Organization names of herbicides approved by the Weed Science Society of for Standardization (ISO). A sponsor may submit a proposal America (WSSA) and updated as of September 1, 2010. for a common name directly to the WSSA Terminology Beginning in 1996, it has been published yearly in the last Committee. issue of Weed Science with Directions for Contributors to A herbicide common name is not synonymous with Weed Science. This list is published in lieu of the selections a commercial formulation of the same herbicide, and in printed previously on the back cover of Weed Science. Only many instances, is not synonymous with the active ingredient common and chemical names included in this complete of a commercial formulation as identified on the product list should be used in WSSA publications. In the absence of label. If the herbicide is a salt or simple ester of a parent a WSSA-approved common name, the industry code number compound, the WSSA common name applies to the parent as compiled by the Chemical Abstracts Service (CAS) with compound only. CAS systematic chemical name or the systematic chemical The chemical name used in this list is that preferred by the name alone may be used. The current approved list is also Chemical Abstracts Service (CAS) according to their system of available at our web site (www.wssa.net). -

Toxicidade De Herbicidas Pós- Emergentes Em Cultivares De Feijão-Caupi
TOXICIDADE DE HERBICIDAS PÓS- EMERGENTES EM CULTIVARES DE FEIJÃO-CAUPI THIAGO REIS PRADO 2016 THIAGO REIS PRADO TOXICIDADE DE HERBICIDAS PÓS-EMERGENTES EM CULTIVARES DE FEIJÃO-CAUPI Dissertação apresentada à Universidade Estadual do Sudoeste da Bahia, Campus de Vitória da Conquista, para obtenção do título de Mestre em Agronomia. Orientador: Prof. D.Sc.Alcebíades Rebouças São José VITÓRIA DA CONQUISTA BAHIA - BRASIL 2016 P915t Prado, Thiago Reis. Toxicidade de herbicidas pós-emergentes em cultivares de feijão-caupi. / Thiago Reis Prado. 55f. Orientador (a): D.Sc. Alcebíades Rebouças São José. Dissertação (mestrado) – Universidade Estadual do Sudoeste da Bahia, Programa de Pós-graduação em Agronomia, área de concentração em Fitotecnia. Vitória da Conquista, 2016. Referências: f. 50-55. 1. Vigna unguiculata - Cultivo. 2. Controle químico. 3. Planta daninha. 4. Fitotoxidade. I. São José, Alcebíades Rebouças II. Universidade Estadual do Sudoeste da Bahia, Programa de Pós-Graduação em Agronomia, área de Concentração em Fitotecnia. III. T. CDD: 633.33 Catalogação na fonte : Juliana Teixeira de Assunção – CRB 5/18 90 UESB – Campus Vitória da Conquista - BA Aos meus pais, Paulo Soares Prado e Maria José Reis Prado, que, sempre dedicados à minha educação e fortes na fé, atuam de forma singular com seus ensinamentos e suas orações. Dedico Agradecimentos A Deus, por iluminar minha vida e guiar por todos os caminhos. Aos meus pais, Paulo Soares Prado e Maria José Reis Prado, pelo amor e por apoiarem sempre as decisões para realização dos meus sonhos. Ao Prof. Dr. Alcebíades Rebouças São José, pela confiança, orientação, compreensão e amizade durante todo o curso do mestrado. -

Glufosinate-Tolerant Cotton: Tolerance and Weed
GLUFOSINATE-TOLERANT COTTON: TOLERANCE AND WEED MANAGEMENT by LESLI KRISTEN BLAIR, B.S. A THESIS IN CROP SCIENCE Submitted to the Graduate Faculty of Texas Tech University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Approved Accepted Deamof the Graduate School December, 1991 ^^•ft:;^ ACKNOWLEDGEMENTS 1i would like to extend my heartfelt gratitude to the members that served on 7 '^ '^* my advisory committee for their time, effort, and assistance. I would like to thank Dr. Dotray for his guidance and direction in the preparation of this thesis; Dr. Keeling for his instruction and support in monitoring these experiments; Dr. Gannaway for his knowledge and assistance in the biotechnology and breeding associated with this project; and Dr. Thompson for her friendship and support in making career decisions. Without all of their guidance, I could never have achieved the goals that we set. I wish to thank all of my fellow graduate students in Weed Science for their help in completing this project. I would especially like to thank Alan Helm for his endless help collecting all of the first year data, Ginger Light for her help in clarifying concepts, and LeAnna Lyon for her help with the last year's project. Their support, friendship, and help on this project have been immeasurable. I would like to express my sincerest thanks to AgrEvo and TxCot for their funding of this research. My thanks also go to the people at the USDA-ARS in Lubbock for all of their help before and after I became involved with this research. -

Herbicide Mode of Action Table High Resistance Risk
Herbicide Mode of Action Table High resistance risk Chemical family Active constituent (first registered trade name) GROUP 1 Inhibition of acetyl co-enzyme A carboxylase (ACC’ase inhibitors) clodinafop (Topik®), cyhalofop (Agixa®*, Barnstorm®), diclofop (Cheetah® Gold* Decision®*, Hoegrass®), Aryloxyphenoxy- fenoxaprop (Cheetah®, Gold*, Wildcat®), fluazifop propionates (FOPs) (Fusilade®), haloxyfop (Verdict®), propaquizafop (Shogun®), quizalofop (Targa®) Cyclohexanediones (DIMs) butroxydim (Factor®*), clethodim (Select®), profoxydim (Aura®), sethoxydim (Cheetah® Gold*, Decision®*), tralkoxydim (Achieve®) Phenylpyrazoles (DENs) pinoxaden (Axial®) GROUP 2 Inhibition of acetolactate synthase (ALS inhibitors), acetohydroxyacid synthase (AHAS) Imidazolinones (IMIs) imazamox (Intervix®*, Raptor®), imazapic (Bobcat I-Maxx®*, Flame®, Midas®*, OnDuty®*), imazapyr (Arsenal Xpress®*, Intervix®*, Lightning®*, Midas®* OnDuty®*), imazethapyr (Lightning®*, Spinnaker®) Pyrimidinyl–thio- bispyribac (Nominee®), pyrithiobac (Staple®) benzoates Sulfonylureas (SUs) azimsulfuron (Gulliver®), bensulfuron (Londax®), chlorsulfuron (Glean®), ethoxysulfuron (Hero®), foramsulfuron (Tribute®), halosulfuron (Sempra®), iodosulfuron (Hussar®), mesosulfuron (Atlantis®), metsulfuron (Ally®, Harmony®* M, Stinger®*, Trounce®*, Ultimate Brushweed®* Herbicide), prosulfuron (Casper®*), rimsulfuron (Titus®), sulfometuron (Oust®, Eucmix Pre Plant®*, Trimac Plus®*), sulfosulfuron (Monza®), thifensulfuron (Harmony®* M), triasulfuron (Logran®, Logran® B-Power®*), tribenuron (Express®), -

Exposure to Herbicides in House Dust and Risk of Childhood Acute Lymphoblastic Leukemia
Journal of Exposure Science and Environmental Epidemiology (2013) 23, 363–370 & 2013 Nature America, Inc. All rights reserved 1559-0631/13 www.nature.com/jes ORIGINAL ARTICLE Exposure to herbicides in house dust and risk of childhood acute lymphoblastic leukemia Catherine Metayer1, Joanne S. Colt2, Patricia A. Buffler1, Helen D. Reed3, Steve Selvin1, Vonda Crouse4 and Mary H. Ward2 We examine the association between exposure to herbicides and childhood acute lymphoblastic leukemia (ALL). Dust samples were collected from homes of 269 ALL cases and 333 healthy controls (o8 years of age at diagnosis/reference date and residing in same home since diagnosis/reference date) in California, using a high-volume surface sampler or household vacuum bags. Amounts of agricultural or professional herbicides (alachlor, metolachlor, bromoxynil, bromoxynil octanoate, pebulate, butylate, prometryn, simazine, ethalfluralin, and pendimethalin) and residential herbicides (cyanazine, trifluralin, 2-methyl-4- chlorophenoxyacetic acid (MCPA), mecoprop, 2,4-dichlorophenoxyacetic acid (2,4-D), chlorthal, and dicamba) were measured. Odds ratios (OR) and 95% confidence intervals (CI) were estimated by logistic regression. Models included the herbicide of interest, age, sex, race/ethnicity, household income, year and season of dust sampling, neighborhood type, and residence type. The risk of childhood ALL was associated with dust levels of chlorthal; compared to homes with no detections, ORs for the first, second, and third tertiles were 1.49 (95% CI: 0.82–2.72), 1.49 (95% CI: 0.83–2.67), and 1.57 (95% CI: 0.90–2.73), respectively (P-value for linear trend ¼ 0.05). The magnitude of this association appeared to be higher in the presence of alachlor. -

WO 2013/037955 Al 21 March 2013 (21.03.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2013/037955 Al 21 March 2013 (21.03.2013) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A01N 25/00 (2006.01) A OIN 43/653 (2006.01) kind of national protection available): AE, AG, AL, AM, A0 41/06 (2006.01) A01N 37/50 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP2012/068096 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 14 September 2012 (14.09.2012) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 1118 1702.9 16 September 201 1 (16.09.201 1) EP (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): BAYER GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, INTELLECTUAL PROPERTY GMBH [DE/DE]; Al- UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, fred-Nobel-Str. -

R Graphics Output
Dexamethasone sodium phosphate ( 0.339 ) Melengestrol acetate ( 0.282 ) 17beta−Trenbolone ( 0.252 ) 17alpha−Estradiol ( 0.24 ) 17alpha−Hydroxyprogesterone ( 0.238 ) Triamcinolone ( 0.233 ) Zearalenone ( 0.216 ) CP−634384 ( 0.21 ) 17alpha−Ethinylestradiol ( 0.203 ) Raloxifene hydrochloride ( 0.203 ) Volinanserin ( 0.2 ) Tiratricol ( 0.197 ) trans−Retinoic acid ( 0.192 ) Chlorpromazine hydrochloride ( 0.191 ) PharmaGSID_47315 ( 0.185 ) Apigenin ( 0.183 ) Diethylstilbestrol ( 0.178 ) 4−Dodecylphenol ( 0.161 ) 2,2',6,6'−Tetrachlorobisphenol A ( 0.156 ) o,p'−DDD ( 0.155 ) Progesterone ( 0.152 ) 4−Hydroxytamoxifen ( 0.151 ) SSR150106 ( 0.149 ) Equilin ( 0.3 ) 3,5,3'−Triiodothyronine ( 0.256 ) 17−Methyltestosterone ( 0.242 ) 17beta−Estradiol ( 0.24 ) 5alpha−Dihydrotestosterone ( 0.235 ) Mifepristone ( 0.218 ) Norethindrone ( 0.214 ) Spironolactone ( 0.204 ) Farglitazar ( 0.203 ) Testosterone propionate ( 0.202 ) meso−Hexestrol ( 0.199 ) Mestranol ( 0.196 ) Estriol ( 0.191 ) 2,2',4,4'−Tetrahydroxybenzophenone ( 0.185 ) 3,3,5,5−Tetraiodothyroacetic acid ( 0.183 ) Norgestrel ( 0.181 ) Cyproterone acetate ( 0.164 ) GSK232420A ( 0.161 ) N−Dodecanoyl−N−methylglycine ( 0.155 ) Pentachloroanisole ( 0.154 ) HPTE ( 0.151 ) Biochanin A ( 0.15 ) Dehydroepiandrosterone ( 0.149 ) PharmaCode_333941 ( 0.148 ) Prednisone ( 0.146 ) Nordihydroguaiaretic acid ( 0.145 ) p,p'−DDD ( 0.144 ) Diphenhydramine hydrochloride ( 0.142 ) Forskolin ( 0.141 ) Perfluorooctanoic acid ( 0.14 ) Oleyl sarcosine ( 0.139 ) Cyclohexylphenylketone ( 0.138 ) Pirinixic acid ( 0.137 ) -

CFS Science Comments I
April 27, 2012 Docket No. APHIS–2010–0103 Regulatory Analysis and Development PPD, APHIS Station 3A-03.8 4700 River Road Unit 118 Riverdale, MD 20737-1238 Comments to USDA APHIS on Environmental Assessment for the Determination of Nonregulated Status of Herbicide-Tolerant DAS-40278-9 Corn, Zea mays, Event DAS- 40278-9 Center for Food Safety, Science Comments I – By Bill Freese, Science Policy Analyst These comments submitted by Center for Food Safety are one of three sets of comments from our organization. Legal comments and a second set of science comments are also being submitted. The references cited have been uploaded as supporting materials. The filenames for these documents match the citations in the text, and are all incorporated as (e.g. Benbrook 2012). Full citations are included at the end of each section. THE IMPACT OF DAS-40278-9 ON CORN HERBICIDE USE Summary of herbicide use Dow’s DAS-402787-9 corn is genetically engineered for resistance to 2,4-D and quizalofop, and if deregulated would be marketed with additional resistance to glyphosate and likely glufosinate – fostering greater use of three to four herbicide classes. APHIS must assess DAS-42078-9 as Dow intends it to be used, as a weed control system. DAS-40278-9 eliminates the risk of crop injury that currently limits 2,4-D use on corn, and is thus reasonably projected to trigger an up to 30-fold increase in the use of this toxic herbicide on corn, equivalent to a four-fold increase in overall agricultural use of 2,4-D, by the end of the decade. -

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

WO 2013/020985 Al 14 February 2013 (14.02.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2013/020985 Al 14 February 2013 (14.02.2013) P O P C T (51) International Patent Classification: (74) Agent: BAYER INTELLECTUAL PROPERTY A 47/06 (2006.01) A01P 7/02 (2006.01) GMBH; Alfred-Nobel-Str. 10, 40789 Monheim (DE). A01N 57/20 (2006.01) A01P 7/04 (2006.01) (81) Designated States (unless otherwise indicated, for every A01P 5/00 (2006.01) kind of national protection available): AE, AG, AL, AM, (21) International Application Number: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, PCT/EP2012/065469 BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 7 August 2012 (07.08.2012) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (25) Filing Language: English ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (30) Priority Data: TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, 61/521,827 10 August 201 1 (10.08.201 1) US ZW. (71) Applicant (for all designated States except US): BAYER (84) Designated States (unless otherwise indicated, for every INTELLECTUAL PROPERTY GMBH [DE/DE]; Al- kind of regional protection available): ARIPO (BW, GH, fred-Nobel-Str. -
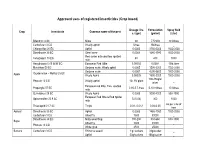
Approved Uses of Registered Insecticides (Crop Based)
Approved uses of registered insecticides (Crop based) Dosage / ha Formulation Spray fluid Crop Insecticide Common name of the pest a.i (gm) (gm/ml) (Liter) Bifenthrin 8 SC Mites 60 7.5ml/lit 10 lit/tree Carbofuran 3 CG Woolly aphid 5/tree 166/tree _ Chlorpyrifos 20 EC Aphid 0.0005 3750-5000 1500-2000 Dimethoate 30 EC Stem borer 0.0003 1485-1980 1500-2000 Red spider mite and two spotted Fenazaquin 10 EC 40 400 1000 mite Hexythiazox 5.45 W/W EC European Red Mite 0.00002 0.0004 10ltr./tree Malathion 50 EC Sanjose scale, Wooly aphid 0.0005 1500-2000 1500-2000 Sanjose scale 0.0007 4200-5600 1500-2000 Oxydemeton – Methyl 25 EC Apple Wooly Aphid 0.00025 1500-2000 1500-2000 100-150gm/ Phorate 10 CG Woolly aphid 10- 15/ plant _ plant European red Mite, Two spotted Propargite 57 EC 2.85-5.7 /tree 5-10 ml/tree 10 lit/tree mite Quinalphos 25 EC Wooly Aphid 0.0005 3000-4000 500-1000 European Red Mite & Red Spider Spiromesifen 22.9 SC 72(0.03) 300 1000 mite As per size of Thiacloprid 21.7 SC Thrips 0.01- 0.012 0.04-0.05 tree Apricot Dimethoate 30 EC Aphid 0.0003 1485-1980 1500-2000 Carbofuran 3 CG Shoot fly 1500 50000 _ Dimethoate 30 EC Milky weed bug 180-200 594-660 500-1000 Bajra Shoot fly 3000 30000 _ Phorate 10 CG White grub 2500 25000 _ Banana Carbofuran 3 CG Rhizome weevil 1 g/ suckers 33g/sucker _ Aphid 50g/suckers 166g/sucker _ Nematode 1.5g/suckers 50g/suckers _ Dimethoate 30 EC Aphid, Lace wing bug 0.0003 1485-1980 1500-2000 Tingyi bug 0.00025 1500-2000 1500-2000 Oxydemeton – Methyl 25 EC Aphids 0.0005 3000-4000 1500-2000 2.5 -1.25/ 25 -12.5/ Phorate 10 CG Aphid _ plant plant Quinalphos 25 EC Tingid bug 0.0005 3000-4000 500-1000 Phosalone 35 EC Aphid 500 1428 500-1000 Aphid 1000 33300 _ Barely Carbofuran 3 CG Jassid 1250 41600 _ Cyst nematode 1000 33300 _ Phorate 10 CG Aphid 1000 10000 _ Beans Chlorpyrifos 20 EC Pod borer , Black bug 600 3000 500-1000 Chlorantraniliprole 18.5 SC Pod borers 25 125 500 Chlorpyrifos 1.5 DP Helicoverpa armigera 375 25000 _ Azadirachtin 0.03 (300 PPM) Pod Borer _ _ _ Bacillus thuringiensis Var.