Evaluating the Effect of Pot Still Design on the Resultant Distillate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Innovation in Continuous Rectification for Tequila Production
processes Communication Innovation in Continuous Rectification for Tequila Production Estarrón-Espinosa Mirna, Ruperto-Pérez Mariela, Padilla-de la Rosa José Daniel * and Prado-Ramírez Rogelio * Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ), Av. Normalistas No. 800, C.P. 44720 Guadalajara, Jalisco, Mexico; [email protected] (E.-E.M.); [email protected] (R.-P.M.) * Correspondence: [email protected] (P.-d.l.R.J.D.); [email protected] (P.-R.R.); Tel.: +33-33455200 (P.-d.l.R.J.D.) Received: 23 March 2019; Accepted: 6 May 2019; Published: 14 May 2019 Abstract: In this study, a new process of continuous horizontal distillation at a pilot level is presented. It was applied for the first time to the rectification of an ordinario fraction obtained industrially. Continuous horizontal distillation is a new process whose design combines the benefits of both distillation columns, in terms of productivity and energy savings (50%), and distillation stills in batch, in terms of the aromatic complexity of the distillate obtained. The horizontal process of continuous distillation was carried out at the pilot level in a manual mode, obtaining five accumulated fractions of distillate that were characterized by gas chromatography (GC-FID). The tequila obtained from the rectification process in this new continuous horizontal distillation process complies with the content of methanol and higher alcohols regulated by the Official Mexican Standard (NOM-006-SCFI-2012). Continuous horizontal distillation of tequila has potential energy savings of 50% compared to the traditional process, besides allowing products with major volatile profiles within the maximum limits established by the regulation for this beverage to be obtained. -

Page2 Sidebar 1.Pdf
Headquarters & Forge Americas Office [email protected] [email protected] +49 (0) 7161 / 97830 215.242.6806 +49 (0) 7161 / 978321 fax fax 215.701.9725 artisan distilling systems 600 liter whiskey still the fine art of distillery technology Germany’s oldest distillery fabricator, since 1869, combining traditional family craftsmanship with leading eau-de-vie distillery innovations and technologies. Meticulously custom-crafted artisan copper pot still systems for all the great distilling traditions, and efficient continuous plants, grappa distillery in copper and stainless steel, for all capacities and applications: 450 liter artisan pot stills – brandy & vodka vodka, whiskey, eaux-de-vie, brandy, rum, gin, grappa, tequila, aguardientes… 1000 liter artisan vodka system 650 liter system with CADi automation continuous mash stripping column C. CARL Ziegelstraße 21 Americas Office Brewing & Distilling Ing. GmbH D-73033 Göppingen PO Box 4388 Technologies Corp. www.christiancarl.com Germany Philadelphia, PA 19118-8388 www.brewing-distilling.com CARL artisan distillery systems the fine art of distillery technology CARL custom-builds each distillery to order in our family shop near Stuttgart in Swabia, with the attention and care of crafting a finely- tuned instrument. All-the-while, we stay focused on the continued development of our distillery technology. There are always new ideas and realizations, such our the in-house developed CARL CADi distillery automation or our patented aroma bubble plate technologies, Our innovations have fostered CARL’s nearly 140 years of family tradition and experience as Germany’s oldest and most respected distillery fabricator, with thousands of successful commissions worldwide. form and function Our diverse customers, from small farmers to winemakers to brewers to large spirits houses, show great enthusiasm and appreciation for the aesthetics and functionality of a CARL distillery: its design, its form, classic and intuitively easy to understand, clear in conception. -

Distillation 6
CHAPTER Energy Considerations in Distillation 6 Megan Jobson School of Chemical Engineering and Analytical Science, The University of Manchester, Manchester, UK CHAPTER OUTLINE 6.1 Introduction to energy efficiency ....................................................................... 226 6.1.1 Energy efficiency: technical issues................................................... 227 6.1.1.1 Heating.................................................................................... 227 6.1.1.2 Coolingdabove ambient temperatures...................................... 229 6.1.1.3 Coolingdbelow ambient temperatures ...................................... 230 6.1.1.4 Mechanical or electrical power.................................................. 232 6.1.1.5 Summarydtechnical aspects of energy efficiency ..................... 233 6.1.2 Energy efficiency: process economics............................................... 233 6.1.2.1 Heating.................................................................................... 233 6.1.2.2 Cooling..................................................................................... 235 6.1.2.3 Summarydprocess economics and energy efficiency ............... 237 6.1.3 Energy efficiency: sustainable industrial development........................ 237 6.2 Energy-efficient distillation ............................................................................... 237 6.2.1 Energy-efficient distillation: conceptual design of simple columns...... 238 6.2.1.1 Degrees of freedom in design .................................................. -

Modelling of Crude Oil Distillation
Modelling of Crude Oil Distillation Modellering av råoljedestillation JENN Y BEATRIC E SOUC K K T H C h em i ca l S ci en c e and E n gi n e e r i n g Master’s Thesis in Chemical Engineering Stockholm, Sweden 2012 " One never notices what has been done; one can only see what remains to be done" (Marie Curie, 1867-1934) 1 Sammanfattning Under de föhållanden som reservoarens miljö erbjuder, definieras en petroleumvätska av dess termodynamiska och volymetriska egenskaper och av dess fysikalisk-kemiska egenskaper. För att korrekt simulera bearbetningen av dessa vätskor under produktion, deras beteende modelleras från experimentella data Med tillkomsten av nya regler och oflexibilitet som finns på tullbestämmelser vid gränserna idag, har forskningscenter stora svårigheter att få större mängder prover levererade. Av den anledningen, trots att det finns flera metoder för att karakterisera de olika komponenterna av råolja, tvingas laboratorier att vända sig mer och mer till alternativa analysmetoder som kräver mindre provvolymer: mikrodestillation, gaskromatografi, etc. Mikrodestillation, som är en snabb och helt datoriserad teknik, visar sig kunna ersätta standarddestillation för analys av flytande petroleumprodukter. Fördelar med metoden jämfört med standarddestillering är minskad arbetstidsåtgång med minst en faktor 4. Därtill krävs endast en [24] begränsad provvolym (några mikroliter) i jämförelse med standarddestillation. Denna rapport syftar till att skapa en enkel modell som kan förutsäga avkastningskurvan av fysisk destillation, utan att använda mikrodestillationsteknik. De resultat som erhölls genom gaskromatografiska analyser möjliggjorde modelleringen av det vätskebeteendet hos det analyserade provet. Efter att ha identifierat och behandlat praktiskt taget alla viktiga aspekter av mikro destillation genom simuleringar med PRO/II, fann jag att, oberoende av inställningen och den termodynamiska metod som används, det alltid finns stora skillnader mellan simulering och mikro destillation. -

The Alcohol Textbook 4Th Edition
TTHEHE AALCOHOLLCOHOL TEXTBOOKEXTBOOK T TH 44TH EEDITIONDITION A reference for the beverage, fuel and industrial alcohol industries Edited by KA Jacques, TP Lyons and DR Kelsall Foreword iii The Alcohol Textbook 4th Edition A reference for the beverage, fuel and industrial alcohol industries K.A. Jacques, PhD T.P. Lyons, PhD D.R. Kelsall iv T.P. Lyons Nottingham University Press Manor Farm, Main Street, Thrumpton Nottingham, NG11 0AX, United Kingdom NOTTINGHAM Published by Nottingham University Press (2nd Edition) 1995 Third edition published 1999 Fourth edition published 2003 © Alltech Inc 2003 All rights reserved. No part of this publication may be reproduced in any material form (including photocopying or storing in any medium by electronic means and whether or not transiently or incidentally to some other use of this publication) without the written permission of the copyright holder except in accordance with the provisions of the Copyright, Designs and Patents Act 1988. Applications for the copyright holder’s written permission to reproduce any part of this publication should be addressed to the publishers. ISBN 1-897676-13-1 Page layout and design by Nottingham University Press, Nottingham Printed and bound by Bath Press, Bath, England Foreword v Contents Foreword ix T. Pearse Lyons Presient, Alltech Inc., Nicholasville, Kentucky, USA Ethanol industry today 1 Ethanol around the world: rapid growth in policies, technology and production 1 T. Pearse Lyons Alltech Inc., Nicholasville, Kentucky, USA Raw material handling and processing 2 Grain dry milling and cooking procedures: extracting sugars in preparation for fermentation 9 Dave R. Kelsall and T. Pearse Lyons Alltech Inc., Nicholasville, Kentucky, USA 3 Enzymatic conversion of starch to fermentable sugars 23 Ronan F. -

Alembic Pot Still
ALEMBIC POT STILL INSTRUCTION MANUAL CAN BE USED WITH THE GRAINFATHER OR T500 BOILER SAFETY Warning: This system produces a highly flammable liquid. PRECAUTION: • Always use the Alembic Pot Still System in a room with adequate ventilation. • Never leave the Alembic Pot Still system unattended when operating. • Keep the Alembic Pot Still system away from all sources of ignition, including smoking, sparks, heat, and open flames. • Ensure all other equipment near to the Alembic Pot Still system or the alcohol is earthed. • A fire extinguishing media suitable for alcohol should be kept nearby. This can be water fog, fine water spray, foam, dry powder, carbon dioxide, sand or dolomite. • Do not boil dry. In the event the still is boiled dry, reset the cutout button under the base of the still. In the very unlikely event this cutout fails, a fusible link gives an added protection. IN CASE OF SPILLAGE: • Shut off all possible sources of ignition. • Clean up spills immediately using cloth, paper towels or other absorbent materials such as soil, sand or other inert material. • Collect, seal and dispose accordingly • Mop area with excess water. CONTENTS Important points before getting started ............................................................................... 3 Preparing the Alembic Pot Still ................................................................................................. 5 Distilling a Whiskey, Rum or Brandy .......................................................................................7 Distilling neutral -

Review of Extractive Distillation. Process Design, Operation
Review of Extractive Distillation. Process design, operation optimization and control Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, Xinqiang You To cite this version: Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, et al.. Review of Extractive Distillation. Process design, operation optimization and control. Chemical Engineering Research and Design, Elsevier, 2019, 141, pp.229-271. 10.1016/j.cherd.2018.09.020. hal-02161920 HAL Id: hal-02161920 https://hal.archives-ouvertes.fr/hal-02161920 Submitted on 21 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive Toulouse Archive Ouverte OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/239894 Official URL: https://doi.org/10.1016/j.cherd.2018.09.020 To cite this version: Gerbaud, Vincent and Rodríguez-Donis, Ivonne and Hegely, Laszlo and Láng, Péter and Dénes, Ferenc and You, Xinqiang Review of Extractive Distillation. Process design, operation optimization and control. (2018) Chemical Engineering Research and Design, 141. -

Computer Simulation of a Multicomponent, Multistage Batch Distillation Process
COMPUTER SIMULATION OF A MULTICOMPONENT, MULTISTAGE BATCH DISTILLATION PROCESS By BRUCE EARL BAUGHER /J Bachelor of Science in Chemical Engineering Oklahoma State University Stillwater, Oklahoma 1985 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE July, 1988 \\,~s~ ~ \'\~~ B~~~to' <:.o?. ·;)._, ~ r COMPUTER SIMULATION OF A MULTICOMPONENT, MULTISTAGE BATCH DISTILLATION PROCESS Thesis Approved: Thesis Advisor U.bJ ~~- ~MJYl~ Dean of the GradU~te College ABSTRACT The material and energy balance equations for a batch distillation column were derived and a computer simulation was developed to solve these equations. The solution follows a modified Newton-Raphson approach for inverting and solving the matrices of material and energy balance equations. The model is unique in that it has been designed to handle hypothetical hydrocarbon components. The simulation can handle columns up to 50 trays and systems of up to 100 components. The model has been used to simulate True Boiling Point (TBP) diagrams for a variety of crude oils. This simulation is also applicable to simple laboratory batch distillations. The model was designed to accurately combine data files to simulate actual crude blending procedures. The model will combine files and calculate the results quickly and easily. The simulation involves removing material from the column at steady-state. The removal fraction is made small enough to approach continuity. This simulation can be adapted for use on microcomputers, although it will require extensive computation time. ACKNOWLEDGMENTS It has been a great pleasure to work with my thesis advisor, Dr. -
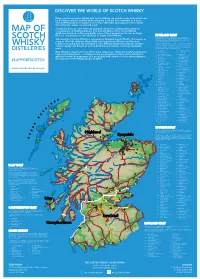
2019 Scotch Whisky
©2019 scotch whisky association DISCOVER THE WORLD OF SCOTCH WHISKY Many countries produce whisky, but Scotch Whisky can only be made in Scotland and by definition must be distilled and matured in Scotland for a minimum of 3 years. Scotch Whisky has been made for more than 500 years and uses just a few natural raw materials - water, cereals and yeast. Scotland is home to over 130 malt and grain distilleries, making it the greatest MAP OF concentration of whisky producers in the world. Many of the Scotch Whisky distilleries featured on this map bottle some of their production for sale as Single Malt (i.e. the product of one distillery) or Single Grain Whisky. HIGHLAND MALT The Highland region is geographically the largest Scotch Whisky SCOTCH producing region. The rugged landscape, changeable climate and, in The majority of Scotch Whisky is consumed as Blended Scotch Whisky. This means as some cases, coastal locations are reflected in the character of its many as 60 of the different Single Malt and Single Grain Whiskies are blended whiskies, which embrace wide variations. As a group, Highland whiskies are rounded, robust and dry in character together, ensuring that the individual Scotch Whiskies harmonise with one another with a hint of smokiness/peatiness. Those near the sea carry a salty WHISKY and the quality and flavour of each individual blend remains consistent down the tang; in the far north the whiskies are notably heathery and slightly spicy in character; while in the more sheltered east and middle of the DISTILLERIES years. region, the whiskies have a more fruity character. -

United States Patent (10) Patent No.: US 9,586,922 B2 Wood Et Al
USOO9586922B2 (12) United States Patent (10) Patent No.: US 9,586,922 B2 Wood et al. (45) Date of Patent: Mar. 7, 2017 (54) METHODS FOR PURIFYING 9,388,150 B2 * 7/2016 Kim ..................... CO7D 307/48 5-(HALOMETHYL)FURFURAL 9,388,151 B2 7/2016 Browning et al. 2007/O161795 A1 7/2007 Cvak et al. 2009, 0234142 A1 9, 2009 Mascal (71) Applicant: MICROMIDAS, INC., West 2010, 0083565 A1 4, 2010 Gruter Sacramento, CA (US) 2010/0210745 A1 8/2010 McDaniel et al. 2011 O144359 A1 6, 2011 Heide et al. (72) Inventors: Alex B. Wood, Sacramento, CA (US); 2014/01 00378 A1 4/2014 Masuno et al. Shawn M. Browning, Sacramento, CA 2014/O1878O2 A1 7/2014 Mikochik et al. (US);US): Makoto N. Masuno,M. s Elk Grove, s 2015/02668432015,0203462 A1 9/20157/2015 CahanaBrowning et etal. al. CA (US); Ryan L. Smith, Sacramento, 2016/0002190 A1 1/2016 Browning et al. CA (US); John Bissell, II, Sacramento, 2016,01681 07 A1 6/2016 Masuno et al. CA (US) 2016/02O7897 A1 7, 2016 Wood et al. (73) Assignee: MICROMIDAS, INC., West FOREIGN PATENT DOCUMENTS Sacramento, CA (US) CN 101475544. A T 2009 (*) Notice: Subject to any disclaimer, the term of this N 1939: A 658 patent is extended or adjusted under 35 DE 635,783 C 9, 1936 U.S.C. 154(b) by 0 days. EP 291494 A2 11, 1988 EP 1049657 B1 3, 2003 (21) Appl. No.: 14/852,306 GB 1220851 A 1, 1971 GB 1448489 A 9, 1976 RU 2429234 C2 9, 2011 (22) Filed: Sep. -

Hydro-Distillation and Steam Distillation from Aromatic Plants
Hydro-distillation and steam distillation from aromatic plants Sudeep Tandon Scientist Chemical Engineering Division CIMAP, Lucknow HISTORY Written records of herbal distillation are found as early as the first century A.D., and around 1000 A.D., the noted Arab physician and naturalist Ibn Sina also known as Avicenna described the distillation of rose oil from rose petals The ancient Arabian people began to study the chemical properties of essential oils & developed and refined the distillation process Europeans began producing essential oils in the 12th century 1 DISTILLATION ? A process in which a liquid or vapour mixture of two or more substances is separated into its component fractions of desired purity, by the application and removal of heat. In simple terms distillation of aromatic herbs implies vaporizing or liberating the oils from the trichomes / plant cell membranes of the herb in presence of high temperature and moisture and then cooling the vapour mixture to separate out the oil from water. It is the most popular widely used and cost effective method in use today for producing majority of the essential oils throughout the world Distillation is an art and not just a “Chemical" process that is reliant upon many factors for successful quality oil production. BASIC SCIENTIFIC PRINCIPLES INVOLVED IN THE PROCESS To convert any liquid into a vapour we have to apply energy in form of heat called as latent heat of vaporization A liquid always boils at the temperature at which its vapour pressure equals the atmospheric / surrounding pressure For two immiscible liquids the total vapour pressure of the mixture is always equal to the sum of their partial pressures The composition of the mixture will be determined by the concentration of the individual components into its partial pressure As known the boiling point of most essential oil components exceeds that of water and generally lies between 150 – 300oC 2 If a sample of an essential oil having a component ‘A’ having boiling point for example 190oC and the boiling point of the water is 100oC. -

Distillation of Alcohol and De-Naturing
The Distillation Group, Inc. Technology in Distillation Distillation of Alcohol And De-Naturing By F. B. Wright 2nd Edition, Published 1907 This converted document: Copyright Andrew W. Sloley 2001. All rights reserved. Copies of this work may be downloaded and installed on electronic data storage systems and in paper format provided: The document is kept complete and is not edited or modified in any way (including by removal of this notice or by deletion or modification of the copyright notice.) PO Box 10105 979-764-3975 College Station, TX 77845 979-764-1449 Fax USA [email protected] www.distillationgroup.com The Distillation Group, Inc. Technology in Distillation Conversion Notes To the greatest extent possible, the original layout of the work has been preserved within the constraints of reasonable conversion to modern electronic format. Exceptions to this (along with some other notes) include: 1. The original text used full width formatting across the page with hyphenation. The converted version uses right justified text and line break hyphenation has been removed. 2. Hyphenation with compound words has been kept. 3. Hyphenation breaks between pages have been kept. 4. Small capitals were originally used in subtitles, figure legends and in some other places. The small capitals have been replaced with regular capitals of the appropriate font size. 5. Spelling and grammar has changed since 1907. Original spelling and grammar was kept. 6. Most mistakes in the original text in spelling, grammar, and punctuation have been kept. 7. Figures that were originally on fold-out plates have been reduced to fit on one page.