Population Dynamics and Movement of Ozark Cavefish in Logan Cave Nwr, Benton County, Arkansas, with Additional Baseline Water Qu
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Preserving the Tree of Life of the Fish Family Cyprinidae in Africa in the Face of the Ongoing Extinction Crisis
Genome Preserving the tree of life of the fish family Cyprinidae in Africa in the face of the ongoing extinction crisis Journal: Genome Manuscript ID gen-2018-0023.R3 Manuscript Type: Article Date Submitted by the 02-Mar-2019 Author: Complete List of Authors: Adeoba, Mariam; University of Johannesburg Tesfamichael, Solomon; University of Johannesburg Yessoufou, Kowiyou; University of Johannesburg Conservation, African freshwater Ecosystems, IUCN Red List, EDGE, DNA Keyword: Draft barcoding Is the invited manuscript for consideration in a Special 7th International Barcode of Life Issue? : https://mc06.manuscriptcentral.com/genome-pubs Page 1 of 42 Genome Preserving the tree of life of the fish family Cyprinidae in Africa in the face of the ongoing extinction crisis Mariam Salami1, Solomon Tesfamichael2, Kowiyou Yessoufou2 1Department of Zoology, University of Johannesburg, Kingsway Campus, PO Box 524, Auckland Park 2006, South Africa 2Department of Geography, Environmental Management and Energy studies, University of Johannesburg, Kingsway Campus, PO Box 524, Auckland Park 2006, South Africa *Corresponding author: Kowiyou Yessoufou [email protected] Draft 1 https://mc06.manuscriptcentral.com/genome-pubs Genome Page 2 of 42 Abstract Our understanding of how the phylogenetic tree of fishes might be affected by the ongoing extinction risk is poor. This is due to the unavailability of comprehensive DNA data, especially for many African lineages. In addition, the ongoing taxonomic confusion within some lineages, e.g. Cyprinidae, makes it difficult to contribute to the debate on how the fish tree of life might be shaped by extinction. Here, we combine COI sequences and taxonomic information to assemble a fully sampled phylogeny of the African Cyprinidae and investigate whether we might lose more phylogenetic diversity (PD) than expected if currently-threatened species go extinct. -

Reproductive Behavior, Development and Eye Regression in the Cave
Neotropical Ichthyology, 7(3):479-490, 2009 Copyright © 2009 Sociedade Brasileira de Ictiologia Reproductive behavior, development and eye regression in the cave armored catfish, Ancistrus cryptophthalmus Reis, 1987 (Siluriformes: Loricariidae), breed in laboratory Sandro Secutti and Eleonora Trajano The troglobitic armored catfish, Ancistrus cryptophthalmus (Loricariidae, Ancistrinae) is known from four caves in the São Domingos karst area, upper rio Tocantins basin, Central Brazil. These populations differ in general body shape and degree of reduction of eyes and of pigmentation. The small Passa Três population (around 1,000 individuals) presents the most reduced eyes, which are not externally visible in adults. A small group of Passa Três catfish, one male and three females, reproduced spontaneously thrice in laboratory, at the end of summertime in 2000, 2003 and 2004. Herein we describe the reproductive behavior during the 2003 event, as well as the early development of the 2003 and 2004 offsprings, with focus on body growth and ontogenetic regression of eyes. The parental care by the male, which includes defense of the rock shelter where the egg clutch is laid, cleaning and oxygenation of eggs, is typical of many loricariids. On the other hand, the slow development, including delayed eye degeneration, low body growth rates and high estimated longevity (15 years or more) are characteristic of precocial, or K-selected, life cycles. In the absence of comparable data for close epigean relatives (Ancistrus spp.), it is not possible to establish whether these features are an autapomorphic specialization of the troglobitic A. cryptophthalmus or a plesiomorphic trait already present in the epigean ancestor, possibly favoring the adoption of the life in the food-poor cave environment. -

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Draft Hunt Plan
Ozark Plateau National Wildlife Refuge White-tailed Deer, Eastern Gray and Fox Squirrel, and Cottontail Rabbit Hunt Plan May 2019 U.S. Fish and Wildlife Service Ozark Plateau National Wildlife Refuge 16602 County Road465 Colcord, Oklahoma 74338-2215 Submitted By: Refuge Manager ______________________________________________ ____________ Signature Date Concurrence: Refuge Supervisor ______________________________________________ ____________ Signature Date Approved: Regional Chief, National Wildlife Refuge System ______________________________________________ ____________ Signature Date i Table of Contents I. Introduction ................................................................................................................................. 1 II. Statement of Objectives ............................................................................................................. 4 III. Description of Hunting Program ............................................................................................... 4 A. Areas to be Opened to Hunting ............................................................................................. 5 B. Species to be Taken, Hunting Periods, Hunting Access ........................................................ 5 C. Hunter Permit Requirements (if applicable) ........................................................................ 12 D. Consultation and Coordination with the State/ Tribes ......................................................... 12 E. Law Enforcement ................................................................................................................ -

Aquatic Fish Report
Aquatic Fish Report Acipenser fulvescens Lake St urgeon Class: Actinopterygii Order: Acipenseriformes Family: Acipenseridae Priority Score: 27 out of 100 Population Trend: Unknown Gobal Rank: G3G4 — Vulnerable (uncertain rank) State Rank: S2 — Imperiled in Arkansas Distribution Occurrence Records Ecoregions where the species occurs: Ozark Highlands Boston Mountains Ouachita Mountains Arkansas Valley South Central Plains Mississippi Alluvial Plain Mississippi Valley Loess Plains Acipenser fulvescens Lake Sturgeon 362 Aquatic Fish Report Ecobasins Mississippi River Alluvial Plain - Arkansas River Mississippi River Alluvial Plain - St. Francis River Mississippi River Alluvial Plain - White River Mississippi River Alluvial Plain (Lake Chicot) - Mississippi River Habitats Weight Natural Littoral: - Large Suitable Natural Pool: - Medium - Large Optimal Natural Shoal: - Medium - Large Obligate Problems Faced Threat: Biological alteration Source: Commercial harvest Threat: Biological alteration Source: Exotic species Threat: Biological alteration Source: Incidental take Threat: Habitat destruction Source: Channel alteration Threat: Hydrological alteration Source: Dam Data Gaps/Research Needs Continue to track incidental catches. Conservation Actions Importance Category Restore fish passage in dammed rivers. High Habitat Restoration/Improvement Restrict commercial harvest (Mississippi River High Population Management closed to harvest). Monitoring Strategies Monitor population distribution and abundance in large river faunal surveys in cooperation -

A Guide to the Parasites of African Freshwater Fishes
A Guide to the Parasites of African Freshwater Fishes Edited by T. Scholz, M.P.M. Vanhove, N. Smit, Z. Jayasundera & M. Gelnar Volume 18 (2018) Chapter 2.1. FISH DIVERSITY AND ECOLOGY Martin REICHARD Diversity of fshes in Africa Fishes are the most taxonomically diverse group of vertebrates and Africa shares a large portion of this diversity. This is due to its rich geological history – being a part of Gondwana, it shares taxa with the Neotropical region, whereas recent close geographical affnity to Eurasia permitted faunal exchange with European and Asian taxa. At the same time, relative isolation and the complex climatic and geological history of Africa enabled major diversifcation within the continent. The taxonomic diversity of African freshwater fshes is associated with functional and ecological diversity. While freshwater habitats form a tiny fraction of the total surface of aquatic habitats compared with the marine environment, most teleost fsh diversity occurs in fresh waters. There are over 3,200 freshwater fsh species in Africa and it is likely several hundreds of species remain undescribed (Snoeks et al. 2011). This high diversity and endemism is likely mirrored in diversity and endemism of their parasites. African fsh diversity includes an ancient group of air-breathing lungfshes (Protopterus spp.). Other taxa are capable of breathing air and tolerate poor water quality, including several clariid catfshes (e.g., Clarias spp.; Fig. 2.1.1D) and anabantids (Ctenopoma spp.). Africa is also home to several bichir species (Polypterus spp.; Fig. 2.1.1A), an ancient fsh group endemic to Africa, and bonytongue Heterotis niloticus (Cuvier, 1829) (Osteoglossidae), a basal actinopterygian fsh. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Ozark Cavefish (Amblyopsis Rosae Eigenmann 1898)
Ozark cavefish (Amblyopsis rosae Eigenmann 1898) Dante Fenolio, Atlanta Botanical Garden 5-Year Review: Summary and Evaluation U.S. Fish and Wildlife Service Southeast Region Arkansas Ecological Services Field Office Conway, Arkansas 5-YEAR REVIEW Ozark cavefish (Amblyopsis rosae) I. GENERAL INFORMATION A. Methodology used to complete review This review was completed by the U.S. Fish and Wildlife Service Arkansas Field Office (AFO) in coordination with the U.S. Fish and Wildlife Service’s Missouri and Oklahoma Field Offices, the Arkansas Game and Fish Commission, the Missouri Department of Conservation, and the Oklahoma Department of Wildlife Conservation. Literature and documents were researched and reviewed as one component of this evaluation. A data table was constructed at the AFO and sent to cavefish biologists currently involved with on-the-ground conservation activities with a request to complete the table and return it to the AFO Ozark cavefish national lead. A second request was made to the same biologists requesting a list of accomplished and ongoing conservation actions. Recommendations resulting from this review are a result of thoroughly reviewing available literature, ongoing conservation actions, input and suggestions from active cavefish biologists, and the reviewers’ expertise on this species. Comments and suggestions regarding the five year review were received from cavefish biologists listed in the peer review section of this document. No part of the review was contracted to an outside party. Special thanks to private landowners, developers, and communities who with their input, support, and cooperative spirit have made Ozark cavefish conservation efforts successful. To respect private and other landowners’ wishes, thereby, not encouraging search of and entry into cavefish locations; cave locations will not be discussed in great detail. -

Etude Comparã©E Du Comportement
Int. J. Speleol. 4 (1972), pp. 139-169. Etude comparee du comportement alimentaire de deux poissons cavernicoles (Anoptichthys jordani Hubbs et Innes et Caecobarbus geertsi Blgr.l par Georges THINES et Nicole WISSOCQ* INTRODUCTION Dans un travail preliminaire pam dans ce meme Journal, Thines, Soffie et Vandenbussche (1966) avaient procede a l'analyse du comportement alimentaire du poisson cavernicole Anoptichthys Gen. et des hybrides F 1 obtenus par croisement Astyanax x Anoptichthys, la premiere espece etant la forme ancestrale epigee de la seconde. Les resultats de ces recherches avaient mis en evidence les traits essentiels de la reaction de la forme cavernicole Anoptichthys a la stimulation chimique. Ces donnees avaient egalement permis de caracteriser la stereotypie d'exploration de ce poisson consecutive a la stimulation chimique initiale. Les auteurs precites avaient etabli que l'exploration alimentaire s'effectuait sous la forme d'une plongee vers Ie fond suivie d'une recherche active localisee autour du point d'impact de la nourriture sur Ie substrat. L'efficacite de saisie, comparee a celIe de la forme ancestrale Astyanax, est tres deficitaire. De plus, la plongee vers Ie fond peut etre declenchee par des stimuli perturbants de nature mecanique. L'ensemble de ces observations avait ete soumis a la discussion et avait amene a conclure que Ie comportement alimentaire, outre les deficits evidents qu'il accuse sur Ie plan de l'efficacite spatio-temporelle de saisie, intervenait a titre substitutif dans des situations declenchant des comportements phobiques typiques chez la forme epigee Astyanax. POSITION DU PROBLEME Si I'on se refere a la classification de Vandel (1964), l'Anoptichthys est un cavernicole recent, tandis que Ie Caecobarbus est un cavernicole ancien. -
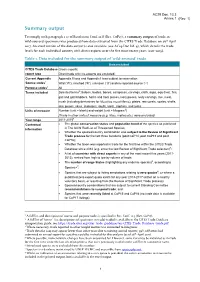
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g. -

Biology of Subterranean Fishes
CHAPTER 7 Subterranean Fishes of North America: Amblyopsidae Matthew L. Niemiller1 and Thomas L. Poulson2 1Department of Ecology and Evolutionary Biology, University of Tennessee, Knoxville, Tennessee, 37996, USA E-mail: [email protected] 2Emeritus Professor, University of Illinois-Chicago E-mail: [email protected] INTRODUCTION The Amblyopsid cavefi shes, family Amblyopsidae, have been viewed as a model system for studying the ecological and evolutionary processes of cave adaptation because the four cave-restricted species in the family represent a range of troglomorphy that refl ect variable durations of isolation in caves (Poulson 1963, Poulson and White 1969). This group has both intrigued and excited biologists since the discovery and description of Amblyopsis spelaea, the fi rst troglobitic fi sh ever described, in the early 1840s. Other than the Mexican cavefi sh (Astyanax fasciatus), cave Amblyopsids are the most comprehensively studied troglobitic fi shes (Poulson, this volume). The Amblyopsidae (Fig. 1) includes species with some unique features for all cavefi sh. Typhlichthys subterraneus is the most widely distributed of any cavefi sh species. Its distribution spans more than 5° of latitude and 1 million km2 (Proudlove 2006). Amblyopsis spelaea is the only cavefi sh known to incubate eggs in its gill chamber. In fact, this species is the only one of the approximately 1100 species in North America with this behavior. The Amblyopsidae is the most specious family of subterranean fi shes in the United States containing four of the eight species recognized. Two other © 2010 by Science Publishers 170 Biology of Subterranean Fishes Fig. 1 Members of the Amblyopsidae. The family includes (A) the surface- dwelling swampfi sh (Chologaster cornuta), (B) the troglophile spring cavefi sh (Forbesichthys agassizii), and four troglobites: (C) the southern cavefi sh (Typhlichthys subterraneus), (D) the northern cavefi sh (Amblyopsis spelaea), (E) the Ozark cavefi sh (A. -

Status Report for the Southern Cavefish, Typhlichthys Subterraneus in Arkansas
City University of New York (CUNY) CUNY Academic Works Publications and Research Baruch College 2007 Status Report for the Southern Cavefish, yphlichthysT subterraneus in Arkansas Aldemaro Romero Jr. CUNY Bernard M Baruch College Michel Conner How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/bb_pubs/296 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Status Report for the Southern Cavefish, Typhlichthys subterraneus in Arkansas Final Report By Aldemaro Romero, Ph.D.* and Michel Conner Department of Biological Sciences Arkansas State University State University, AR 72467, USA *[email protected] Submitted to the Arkansas Game and Fish Commission 22 July 2007 2 Executive Summary This preliminary report summarizes the results of our study on the status of the southern cavefish (Typhlichthys subterraneus) in Arkansas. Its presence in the state represents the western- southern limits of its distribution. Three localities have been confirmed that contain individuals of this species: Richardson Cave (Fulton County), Alexander Cave/ Clark Spring (Stone County) and Ennis Cave (Stone County). A fourth locality has been cited as a well in Randolph County, but because the exact location is unknown, its presence has not been confirmed. There are a number of unconfirmed localities for “cavefishes” in the region which are listed in this report. Populations of this species in Arkansas seem to be small (less than 100 individuals) which is common among populations of hypogean amblyopsids elsewhere. All the confirmed localities are in areas either under controlled access by the private owners or by the federal government.