The Camborne Mining District in 1904 a View Taken from the Engine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LCAA8361 Guide £525000 4 Trencrom Row, Trencrom, Lelant Downs, Nr. St Ives, Cornwall, TR27 6NU FREEHOLD Situated
Ref: LCAA8361 Guide £525,000 4 Trencrom Row, Trencrom, Lelant Downs, Nr. St Ives, Cornwall, TR27 6NU FREEHOLD Situated in a stunning, elevated position enjoying panoramic views of the surrounding undulating countryside towards St Ives bay; an extremely well presented 3 double bedroomed extended former miner’s cottage, positioned within an Area of Outstanding Natural Beauty and Site of Special Scientific Interest close to the north Cornish coastline. An impeccably presented home in a little known, highly desirable setting offering semi-rural peace and tranquillity, yet within easy reach of West Cornwall’s large harbourside towns of St Ives, Hayle and Penzance. 2 Ref: LCAA8361 SUMMARY OF ACCOMMODATION – In all, about 1,304sq.ft. Ground Floor: entrance lobby, large sitting/dining room, stunning kitchen/breakfast room with wall of bi-fold doors leading to the private rear garden. First Floor: 2 double bedrooms, family bathroom. Top Floor: principal en-suite double bedroom. Outside: low maintenance enclosed courtyard garden to front and beautifully landscaped, private, west facing rear garden. DESCRIPTION • No. 4 represents the ideal combination of an exquisitely presented home, in a semi- rural setting, surrounded by miles of open farmland, yet with convenient access into some of West Cornwall’s largest towns including St Ives, Hayle and Penzance. • Trencrom Row is an attractive late 1800’s terrace of former miner’s cottages, originally built as part of Trencrom Mine. The National Trust owned ‘Trencrom Hill’ is an Iron Age settlement/hill fort, now designated as both an Area of Outstanding 3 Ref: LCAA8361 Natural Beauty and Site of Special Scientific Interest with the St Michael’s Way (Coast to Coast route) passing nearby. -

Mr. Ellis Crompton-Brown Planning Officer Ref:- PA15/05327 Planning and Enterprise Service Cornwall Council Circuit House Pydar Street Truro TR1 1ES
Mr. Ellis Crompton-Brown Planning Officer Ref:- PA15/05327 Planning and Enterprise Service Cornwall Council Circuit House Pydar Street Truro TR1 1ES Dear Mr. Crompton - Brown I write to state my support to the proposed solar PV development, associated landscaping and habitat creation, at land Immediately East of Junction between Pilgrims Way and Bunkers Hill Pilgrims Way Fraddam Cornwall, application planning number PA15/05327. I understand that the Appeal Inspector and the Secretary of State have considered all the planning policies relating to this proposal and found them all met with the exception of one. However having considered the only reason for refusal and I feel that the amendments provided by the applicant more than cover the Appeal Inspectors and the Secretary of States concern about amenity at the residences in the North West corner of the proposed site. This is a good site for such a Solar Development as it is accepted by both the Appeal Planning Inspector and the Secretary of State that it is discrete within the landscape, and has very limited views by the public on a daily basis. There is a firm belief, that if the Mably best practice ethos was available four years ago, there would be fewer but better quality solar developments within Cornwall, as many would not have met such exacting criteria. Yours faithfully Mably Solar Amended Planning Application Number PA15/05327 Amended Application with development pulled back Original Application Refused permission due to proximity to Residences in North West Corner into the field within the topography and with guidance of Cornwall Council Planning department 1. -

Minewater Study
National Rivers Authority (South Western-Region).__ Croftef Minewater Study Final Report CONSULTING ' ENGINEERS;. NATIONAL RIVERS AUTHORITY SOUTH WESTERN REGION SOUTH CROFTY MINEWATER STUDY FINAL REPORT KNIGHT PIESOLD & PARTNERS Kanthack House Station Road September 1994 Ashford Kent 10995\r8065\MC\P JS TN23 1PP ENVIRONMENT AGENCY 125218 r:\10995\f8065\fp.Wp5 National Rivers Authority South Crofty Minewater Study South Western Region Final Report CONTENTS Page EXECUTIVE SUMMARY -1- 1. INTRODUCTION 1-1 2. THE SOUTH CROFTY MINE 2-1 2.1 Location____________________________________________________ 2-1 ________2.2 _ Mfning J4istojy_______________________________________ ________2-1. 2.3 Geology 2-1 2.4 Mine Operation 2-2 3. HYDROLOGY 3-1 3.1 Groundwater 3-1 3.2 Surface Water 3-1 3.3 Adit Drainage 3-2 3.3.1 Dolcoath Deep and Penhale Adits 3-3 3.3.2 Shallow/Pool Adit 3-4 3.3.3 Barncoose Adit 3-5 4. MINE DEWATERING 4-1 4.1 Mine Inflows 4-1 4.2 Pumped Outflows 4-2 4.3 Relationship of Rainfall to Pumped Discharge 4-3 4.4 Regional Impact of Dewatering 4-4 4.5 Dewatered Yield 4-5 4.5.1 Void Estimates from Mine Plans 4-5 4.5.2 Void Estimate from Production Tonnages 4-6 5. MINEWATER QUALITY 5-1 5.1 Connate Water 5-2 5.2 South Crofty Discharge 5-3 5.3 Adit Water 5-4 5.4 Acidic Minewater 5-5 Knif»ht Piesold :\10995\r8065\contants.Wp5 (l) consulting enCneers National Rivers Authority South Crofty Minewater Study South Western Region Final Report CONTENTS (continued) Page 6. -

5154 the London Gazette, 15™ April 1977
5154 THE LONDON GAZETTE, 15™ APRIL 1977 Proofs—29th April, 1977. Name of Trustee and Address Name of Trustee, Grimwood, Victor Ernest. Address—^ —Armitage, Anthony John-, Tower House, Merrion Way, Central Chambers, Chartered Accountants, 76, High Leeds 2. Street, Sittingbourne, Kent. .TITHECOTT, Vernon Arthur, and TITHECOTT, ROBERTS, Eiril John, of 4, Ffordd Aneurin, Pontyberem Jennifer (his wife), residing and carrying on business as in the county of Dyfed, lately residing at 86, Maesglas, partnership at 126, Beaumont Road, St. Judes, Plymouth, Pontyates, Dyfed aforesaid, unemployed. Court—SWAN- as FISH and CHIP SHOP PROPRIETORS, previously SEA. No. of Matter—3 of 1975. Last Day for Receiv- carrying on business at " Victory Fish and Chip Shop", ing Proofs—3rd May, 1977. Name of Trustee and Farm Lane, Honicknowle, Plymouth and previously Address—Nunn, Philip William, 5th Floor, Sun Alliance residing at 53, Doone Way, Ilfracombe, all in the county House, 166-167, St. Helen's Road, Swansea, SA1 SDL, of Devon, (Joint Estate.) Court—PLYMOUTH. N*>. of Official Receiver. Matter—58 of 1974. Last Day for Receiving Proofs— TREMEER, Ronald George, of 120, Gwelfor, Killay, Swan- 29th April, 1977. Name of Trustee and Address—Seldon, sea in the county of West Glamorgan, CIVIL SERVANT. Arthur John, 1st Floor, 63, New George Street, Plymouth, Court—SWANSEA. No. of Matter—21 of 1975. Last Devon, Official Receiver. Day for Receiving Proofs—29th April, 1977. Name of Trustee and Address—Down, Gordon Harold, 106, Walter LAWRENCE, George Macdonald, carrying on business as Road, Swansea, SA1 5QX. Elson Engineering Co., of 110A, Whitley Street, Read- ing in the county of Berks, and residing at 4, Rowley THOMAS, Barnes Cravath, and THOMAS, Barbara June, Road, Reading, Berks., MANUFACTURING EN- of Trencrom Nurseries, Lelant Downs, Hayle in the GINEER. -

Notice of Poll and Situation of Polling Stations
NOTICE OF POLL AND SITUATION OF POLLING STATIONS CORNWALL COUNCIL VOTING AREA Referendum on the United Kingdom's membership of the European Union 1. A referendum is to be held on THURSDAY, 23 JUNE 2016 to decide on the question below : Should the United Kingdom remain a member of the European Union or leave the European Union? 2. The hours of poll will be from 7am to 10pm. 3. The situation of polling stations and the descriptions of persons entitled to vote thereat are as follows : No. of Polling Station Situation of Polling Station(s) Description of Persons entitled to vote 301 STATION 2 (AAA1) 1 - 958 CHURCH OF JESUS CHRIST OF LATTER-DAY SAINTS KINGFISHER DRIVE PL25 3BG 301/1 STATION 1 (AAM4) 1 - 212 THE CHURCH OF JESUS CHRIST OF LATTER-DAY SAINTS KINGFISHER DRIVE PL25 3BG 302 CUDDRA W I HALL (AAA2) 1 - 430 BUCKLERS LANE HOLMBUSH ST AUSTELL PL25 3HQ 303 BETHEL METHODIST CHURCH (AAB1) 1 - 1,008 BROCKSTONE ROAD ST AUSTELL PL25 3DW 304 BISHOP BRONESCOMBE SCHOOL (AAB2) 1 - 879 BOSCOPPA ROAD ST AUSTELL PL25 3DT KATE KENNALLY Dated: WEDNESDAY, 01 JUNE, 2016 COUNTING OFFICER Printed and Published by the COUNTING OFFICER ELECTORAL SERVICES, ST AUSTELL ONE STOP SHOP, 39 PENWINNICK ROAD, ST AUSTELL, PL25 5DR No. of Polling Station Situation of Polling Station(s) Description of Persons entitled to vote 305 SANDY HILL ACADEMY (AAB3) 1 - 1,639 SANDY HILL ST AUSTELL PL25 3AW 306 STATION 2 (AAG1) 1 - 1,035 THE COMMITTEE ROOM COUNCIL OFFICES PENWINNICK ROAD PL25 5DR 306/1 STATION 1 (APL3) 1 - 73 THE COMMITTEE ROOM CORNWALL COUNCIL OFFICES PENWINNICK -

Tappard Farm Barns Deveral Road, Fraddam, Hayle, Cornwall Tr27 5Ep
Ref: LAT210022 GUIDE PRICE: £295,000 A Prime Development Opportunity in Rural Surroundings TAPPARD FARM BARNS DEVERAL ROAD, FRADDAM, HAYLE, CORNWALL TR27 5EP A collection of former farm barns with residential planning consent to create a small and appealing development of three homes together with additional sheds ideal for storage with possible further potential. Tucked away on the rural fringes of popular West Cornwall villages, this sheltered location is easily accessible to the nearby towns of Hayle and Helston. HAYLE (A30) 3.5 MILES * HELSTON 7 MILES * CAMBORNE 5 MILES TRURO 22 MILES * FALMOUTH 17 MILES SITUATION Barely a minute from the B3302 Hayle to Helston Road, this is an extremely central and convenient setting hidden away from most day to day hustle and bustle. The communities of Fraddam, Reawla, Wall, Carnhell Green and Leedstown are all within a 3 mile radius. There is a Post Office/grocery store in Carnhell Green with a more extensive range of shops, doctors’ surgery, dentists and a hospital less than 4 miles away within the north coastal harbour town of Hayle which also boasts a choice of supermarkets and a retail park with Marks & Spencer and Boots etc. The County’s main arterial route, the A30, bypasses Hayle providing easy access to Penzance in the west and the City of Truro in the east and the town also has a station on the main Penzance to Paddington railway line. THE BARNS A courtyard of former farm barns and sheds with Conditional Planning Permission (PA18/03716) for residential conversion into three character homes. Additional useful buildings would be ideal for general storage of building materials and/or vehicles and equipment etc, and may also present potential for alternative use, subject to planning consent. -

CORNWALL Hender W. St. Thomas Hill, Launceston Hicks S
190 CORNWALL POST FARMERs-continued. Hender W. St. Thomas hill, Launceston Hicks S. Lewanick, Launceston Hawken G.L. Dannonchapple,f:t.Teath, Hendy A. Trebell, Lanivet, Bodmin Hicks T. Carn, Lelant, Hay le Camelford Hendy E. Trebell, Lanivet, Bodmin Hicks T. Chynalls, St. Paul, Penzance Hawken H. Trefresa, Wadebridge Hendy H. Carmina, Mawgan, Helston Hicks T. Sancreed, Peuzance *Haw ken J.Penrose,St.Ervan, Padstow Hendy J. Trethurffe, Ladock,Grmpound Hicks T. Prideaux, Luxulion, Bodmin Hawken J. Treginnegar, Padstow Hendy J. Frogwell, Callington Hicks T. St. Autbony, Tre~ony HawkenJ.Treburrick,St.Ervan,Padstow Hendy J. Skewes, Cury, Helston Hicks T. Lanivet, Bodmin Haw ken J. jun. Penro~e, Pads tow Hendy J. Frowder, Mullion, Helston Hick;~ T. St. Gerrans, Gram pound Hawken N. Treore, Wadebridge Hendy M. Swyna, Gunwallot>, Helston Hicks T. St. Gennys, Camt>lford Haw ken P. Longcarne, Camelt'ord Hendy S. GunwalloP, Helston Hicks T.jun. Tregarneer,St.Colmb.Major Haw ken P.Tre~wyn, St. Ervan,Padstow Hendy T. Lizard, Helston Hicks W. Clift' farm, Anthony Haw ken R. Stanon,St.Breward, Bodmin Hendy W. Chimber, Gunwalloe,Helston Hicks W. St. Agnes, Scilly HawkenR.G.Trt-gwormond,Wadebrilige Hendy W. Mullion, Ht-lston Hicks W. Newlyn East, Grampound HawkenS.Low.Nankelly,St.ColumhMjr Ht>ndy W. PolJ(reen, Cury, Helston Hicks W. PencrebPr farm, Caliington Hawken T. Hale, St. Kew, Wadebridge Hendy W. Polgreen,Gunwalloe, Helston Hicks W. Fowey, Lostwithiel Haw ken T. Heneward, Bolimin Hermah H. Penare, Gorran, St. A ustell Hicks W. St. Agnes, Scilly Haw ken T. Trevorrick, St.lssry ,Bodmin Hennah T. -
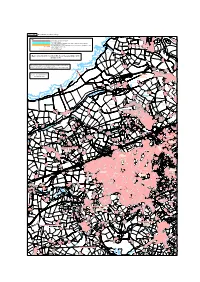
Map Referred to in the Cornwall (Electoral Changes) Order 2011 D R a I Lw a Sheet 5 of 20 Y
SHEET 5, MAP 5 Electoral Divisions in Camborne and Ilogan Park Portreath 1 0 3 3 KEY B L L ELECTORAL DIVISION BOUNDARY Carvannel Downs I H A PARISH BOUNDARY E G E R PARISH BOUNDARY COINCIDENT WITH ELECTORAL DIVISION BOUNDARY T PARISH WARD BOUNDARY Feadon Farm PARISH WARD BOUNDARY COINCIDENT WITH ELECTORAL DIVISION BOUNDARY MOUNT HAWKE AND PORTREATH ED ILLOGAN ED ELECTORAL DIVISION NAME CAMBORNE CP PARISH NAME PORTREATH CP TEHIDY PARISH WARD PARISH WARD NAME 1 0 3 3 B Carvannel Carvannel Farm Downs Mirrose Chytodden D is Well Cove m a n t le Map referred to in the Cornwall (Electoral Changes) Order 2011 d R a i lw a Sheet 5 of 20 y Penpraze Trengove Crane Islands Basset's Cove This map is based upon Ordnance Survey material with the permission of Ordnance Survey on behalf of the Controller of Her Majesty's Stationery Office © Crown copyright. Tehidy Barton Unauthorised reproduction infringes Crown copyright and may lead to prosecution or civil proceedings. The Local Government Boundary Commission for England GD100049926 2011. Nursery r e t r a e t W a w W o ILLOGAN ED h A L LE Scale : 1cm = 0.08500 km g XA n i ND a RA H R e OA n D M a ILLOGAN Grid interval 1km e M PARISH WARD Golf Links C O T R Greenbank Cove O A NE D LA fs E lif IN C B h D rt OO o W N ILLOGAN CP Deadman's Cove Reskajeage Downs (National Trust) S P A R LA N E S O U T H D R Old Merrose Farm IV E I Derrick LL Merrose Farm O Cove iffs G Cl A rth N No D O W N S Home Farm Tehidy Park 01 B 33 TEHIDY Nursery PARISH WARD PARK BOTTOM PARISH WARD Downs Farm Magor Farm POOL AND TEHIDY -

Ref: LCAA6203 £485,000
Ref: LCAA6203 £485,000 Ashmeadow Barn, Trenerth, Fraddam, Hayle, Cornwall FREEHOLD A most impressive single storey 4 bedroomed, 4 bath/shower roomed contemporary converted barn in gardens of about ⅓ of an acre set in a delightful rural former farming hamlet. A wonderful countryside home with generous parking, workshops, sundeck, large paved terrace and private garden, that is still very convenient for Hayle, the north and south Cornish coasts and has excellent road connections nearby. 2 Ref: LCAA6203 SUMMARY OF ACCOMMODATION Entrance hall, kitchen/dining room, living room, summer sitting room, utility, long inner hall, 4 bedrooms (2 en-suite), contemporary wet shower room, family bath/shower room. Outside: About ⅓ of an acre of mostly very private lawned gardens with a large terrace, sundeck and growing beds. Very large modern timber workshop, carport, glasshouse and garden shed plus two generous parking areas. DESCRIPTION Converted in 2001 and greatly updated and extended since Ashmeadow Barn is a very attractive granite and random stone faced extensive single story barn conversion in a rural but not isolated former farming hamlet. Inside there are four double bedrooms, two of which are en-suite, and there is also a family bathroom and separate 3 Ref: LCAA6203 contemporary wet shower room with three of these facilities having under floor heating. These rooms and an excellent utility serve a kitchen/dining room which opens through to a cosy living room with woodburning stove and there is also a further large reception room with bi-fold doors to the rear garden and a glass atrium filling it with light. -

Wave Hub Appendix N to the Environmental Statement
South West of England Regional Development Agency Wave Hub Appendix N to the Environmental Statement June 2006 Report No: 2006R001 South West Wave Hub Hayle, Cornwall Archaeological assessment Historic Environment Service (Projects) Cornwall County Council A Report for Halcrow South West Wave Hub, Hayle, Cornwall Archaeological assessment Kevin Camidge Dip Arch, MIFA Charles Johns BA, MIFA Philip Rees, FGS, C.Geol Bryn Perry Tapper, BA April 2006 Report No: 2006R001 Historic Environment Service, Environment and Heritage, Cornwall County Council Kennall Building, Old County Hall, Station Road, Truro, Cornwall, TR1 3AY tel (01872) 323603 fax (01872) 323811 E-mail [email protected] www.cornwall.gov.uk 3 Acknowledgements This study was commissioned by Halcrow and carried out by the projects team of the Historic Environment Service (formerly Cornwall Archaeological Unit), Environment and Heritage, Cornwall County Council in partnership with marine consultants Kevin Camidge and Phillip Rees. Help with the historical research was provided by the Cornish Studies Library, Redruth, Jonathan Holmes and Jeremy Rice of Penlee House Museum, Penzance; Angela Broome of the Royal Institution of Cornwall, Truro and Guy Hannaford of the United Kingdom Hydrographic Office, Taunton. The drawing of the medieval carved slate from Crane Godrevy (Fig 43) is reproduced courtesy of Charles Thomas. Within the Historic Environment Service, the Project Manager was Charles Johns, who also undertook the terrestrial assessment and walkover survey. Bryn Perry Tapper undertook the GIS mapping, computer generated models and illustrations. Marine consultants for the project were Kevin Camidge, who interpreted and reported on the marine geophysical survey results and Phillip Rees who provided valuable advice. -

Godrevy Cove
North Coast – West Cornwall GODREVY COVE This is stretch of beach at low water forms the northern end of the longest beach in Cornwall (5.5km) sweeping round St.Ives Bay to the Hayle Estuary. For most people the beach starts at the Red River and continues to the headland. Facing due west it has views of St.Ives and the Penwith Moors beyond. The sandy beach above high water mark Cove with steps to the beach. At high water there is only a small area of fine golden sand but at low water the beach stretches for over 700m, interspersed with rocky outcrops, to the Red River where it joins the beach of Gwithian. In winter, much of the sand can often be replaced by areas of shingle. The beach can be quite exposed both from any wind from a westerly direction and also the Atlantic swell. Immediately north of the sandy Cove there is an accessible rocky foreshore with patches of The Cove with the iconic Godrevy Island and Lighthouse beyond shingle which is worth exploring but care needs to be taken not to be caught by an incoming tide TR27 5ED - The access road to the National Trust car parks is 1km north of Gwithian on There is rescue/safety equipment and RNLI the B3301 coast road from Hayle to Portreath by the lifeguards are on duty at the Red River end of the bridge over the Red River. The main car parking area beach from mid May until the end of September. (capacity over 100 cars) is open all year, on the edge of the sand dunes, and, within a short walk to the beach along a fenced board-walk path. -

Cobalt Mineralisation in Cornwall – a New Discovery at Porthtowan
G.K. Rollinson, N. Le Boutillier and R. Selly COBALT MINERALISATION IN CORNWALL – A NEW DISCOVERY AT PORTHTOWAN G.K. ROLLINSON 1, N. LE BOUTILLIER AND R. SELLY Rollinson, G.K., Le Boutillier, N. and Selly, R. 2018. Cobalt mineralisation in Cornwall – A new discovery at Porthtowan. Geoscience in South-West England, 14, 176–187. Although cobalt mineralisation has been noted in Cornwall and Devon in the mining literature, there are limited details of its production and paragenesis; detailed mineral studies of cobalt are almost non-existent. This paper describes in detail previously unrecorded cobalt mineralisation discovered at Porthtowan, Cornwall, in the vicinity of old workings which are part of the Wheal Lushington group of mines, immediately west of the village. A small number of massive sulphide/gangue samples (taken from a larger sample suite) were chosen to be as representative as possible. Analysis was carried out using a QEMSCAN® automated mineral SEM-EDS system, which found that samples contained up to 50% cobaltite, along with chalcopyrite, bornite, galena, sphalerite, acanthite, erythrite, matildite, chlorargyite and other primary and secondary mineral species. This assemblage is typical of a sub-type of crosscourse mineralisation, with secondary species a result of significant weathering and supergene alteration, complicated by seawater infiltration due to the coastal location. While the number of samples is limited, the detail of the mineralogical assemblage is significant, as it is the first time such an assemblage has been subjected to this level of scientific scrutiny in Cornwall. 1 Camborne School of Mines, University of Exeter, Cornwall Campus, Penryn, Cornwall, TR10 9FE, UK.