Type II Spiral Ganglion Afferent Neurons Drive Medial Olivocochlear Reflex Suppression of the Cochlear Amplifier
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fibroblast Growth Factor (Fgf) Implication in the Neonatal Development of the Cochlear Innervation G
FIBROBLAST GROWTH FACTOR (FGF) IMPLICATION IN THE NEONATAL DEVELOPMENT OF THE COCHLEAR INNERVATION G. Després, I. Jalenques, R. Romand To cite this version: G. Després, I. Jalenques, R. Romand. FIBROBLAST GROWTH FACTOR (FGF) IMPLICATION IN THE NEONATAL DEVELOPMENT OF THE COCHLEAR INNERVATION. Journal de Physique IV Proceedings, EDP Sciences, 1992, 02 (C1), pp.C1-173-C1-176. 10.1051/jp4:1992134. jpa-00251205 HAL Id: jpa-00251205 https://hal.archives-ouvertes.fr/jpa-00251205 Submitted on 1 Jan 1992 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. JOURNAL DE PHYSIQUE IV Colloque C1, suppICment au Journal de Physique 111, Volume 2, avril 1992 FIBROBLAST GROWMI FACTOR (FGF) IMPLICATION IN TIlE NEONATAL DEVELOPMENT OF THE COCIiLEAR INNERVATION G. DESPR~S,I. JALENQUES and R. ROMAND Laboratoire de Neurobiolc@e et Physwlogie du Dkeloppement, Universite'BIaise Pascal, F-63177Aubi2re ceder, France The presence of fibroblast growth factor-like protein has been investigated on cryostat sections from Sprague Dawley rat cochleae and auditory brainstem nuclei of various neonatal stages by indirect immunofluorescence and immunoperoxydase techniques with an antibody directed against the 1-24 amino-acid sequence of brain derived basic FGF. -

A Guide to Otoacoustic Emissions Contents
Science made smarter A Guide to Otoacoustic Emissions Contents What are OAEs? ................................................................................ 4 Stimulus tolerance............................................................................................................................................................22 Filtering (TEOAE) ................................................................................................................................................................22 What are Otoacoustic Emissions (OAEs)? ........................................................... 4 Noise rejection level ......................................................................................................................................................23 Types of OAEs ................................................................................................................ 5 Measuring at ambient or tympanic peak pressure (pressurized OAE) ...........................................23 DPOAEs ............................................................................................................................. 6 OAE detection/stopping criteria ............................................................................................................................25 TEOAEs .............................................................................................................................. 7 Screening vs diagnostic OAE ........................................................8 Before and -

5.1. Structure of the Spiral Ganglion
CHAPTER 5. INNERVATION OF THE ORGAN OF CORTI The investigation of nerve components of the acoustic system’s periph- eral part is so difficult methodologically that a whole series of questions which were solved long ago during the investigation of the other sensory system have not yet been clarified. The basic difficulty for the morphologists lies in the fact that the organ of Corti, together with its nerve elements, is located within the osseous tissue. In addition, it is in the shape of a spirally involut- ed geometrical figure. These structural peculiarities create considerable dif- ficulties during the determination of the connections between the types of peripheral and central neuron’s processes and bodies of the spiral ganglion of the cochlea. Therefore, most of the work on the cochlea’s innervation and the computation of the different element’s quantity demands an application of special methods, including graphical reconstruction of the serial sections. The Golgi method was and still remains the basic histological method of the organ of Corti’s innervation study, which has been supplemented by the cochlea’s electron-microscope investigations in the normal conditions and during the experimentally induced degenerations. 5.1. Structure of the spiral ganglion The neurons which innervate the auditory receptor cells form a spiral ganglion: a nerve-knot of the VIII pair’s acoustic part of the craniocerebral nerves. The ganglion fills the Rosental’s canal in the cochlea’s axis and re- peats the number of its spiral turns. The ganglionic neuron has, as a rule, a widened body with two processes: peripheral and central (Diagram 7). -

Auditory Neuropathy After Damage to Cochlear Spiral Ganglion Neurons
www.nature.com/scientificreports OPEN Auditory Neuropathy after Damage to Cochlear Spiral Ganglion Neurons in Mice Resulting from Conditional Received: 27 July 2016 Accepted: 15 June 2017 Expression of Diphtheria Toxin Published online: 25 July 2017 Receptors Haolai Pan1, Qiang Song1, Yanyan Huang1, Jiping Wang1, Renjie Chai2, Shankai Yin1 & Jian Wang 1,3 Auditory neuropathy (AN) is a hearing disorder characterized by normal cochlear amplifcation to sound but poor temporal processing and auditory perception in noisy backgrounds. These defcits likely result from impairments in auditory neural synchrony; such dyssynchrony of the neural responses has been linked to demyelination of auditory nerve fbers. However, no appropriate animal models are currently available that mimic this pathology. In this study, Cre-inducible diphtheria toxin receptor (iDTR+/+) mice were cross-mated with mice containing Cre (Bhlhb5-Cre+/−) specifc to spiral ganglion neurons (SGNs). In double-positive ofspring mice, the injection of diphtheria toxin (DT) led to a 30–40% rate of death for SGNs, but no hair cell damage. Demyelination types of pathologies were observed around the surviving SGNs and their fbers, many of which were distorted in shape. Correspondingly, a signifcant reduction in response synchrony to amplitude modulation was observed in this group of animals compared to the controls, which had a Cre− genotype. Taken together, our results suggest that SGN damage following the injection of DT in mice with Bhlhb5-Cre+/− and iDTR+/− is likely to be a good AN model of demyelination. Auditory neuropathy (AN) is a hearing disorder characterized as having normal cochlear microphonic (CM) potentials and otoacoustic emissions (OAEs), but largely reduced or missing auditory brainstem responses (ABRs). -

Activity-Dependent Regulation of Prestin Expression in Mouse Outer Hair Cells
J Neurophysiol 113: 3531–3542, 2015. First published March 25, 2015; doi:10.1152/jn.00869.2014. Activity-dependent regulation of prestin expression in mouse outer hair cells Yohan Song, Anping Xia, Hee Yoon Lee, Rosalie Wang, Anthony J. Ricci, and John S. Oghalai Department of Otolaryngology-Head and Neck Surgery, Stanford University, Stanford, California Submitted 4 November 2014; accepted in final form 19 March 2015 Song Y, Xia A, Lee HY, Wang R, Ricci AJ, Oghalai JS. patch-clamp recording conditions (Kakehata and Santos-Sac- Activity-dependent regulation of prestin expression in mouse outer chi, 1995 and 1996; Santos-Sacchi 1991; Santos-Sacchi et al. hair cells. J Neurophysiol 113: 3531–3542, 2015. First published 1998a). Thus, measuring the NLC is a common way of assess- March 25, 2015; doi:10.1152/jn.00869.2014.—Prestin is a membrane protein necessary for outer hair cell (OHC) electromotility and normal ing the amount of functional prestin within an OHC (Abe et al. 2007; Oliver and Fakler 1999;). hearing. Its regulatory mechanisms are unknown. Several mouse Downloaded from models of hearing loss demonstrate increased prestin, inspiring us to There are many ways to modulate the voltage dependence of investigate how hearing loss might feedback onto OHCs. To test force production by prestin protein within the plasma mem- whether centrally mediated feedback regulates prestin, we developed brane, such as changing the surrounding lipid environment, a novel model of inner hair cell loss. Injection of diphtheria toxin (DT) intracellular Ca2ϩ level, membrane tension, membrane poten- into adult CBA mice produced significant loss of inner hair cells tial, and ionic composition of the intracellular and extracellular without affecting OHCs. -

Introduction to Sound and Hearing
Salamanca Study Abroad Program: Neurobiology of Hearing Cochlear mechanics R. Keith Duncan University of Michigan [email protected] Outline Passive and active cochlear mechanics Prestin and outer hair cell motility Tonotopicity A place code is a general principle of many sensory systems (somatotopic, retinotopic, others?) Damage (hair cell loss) at particular locations along the cochlea is correlated with hearing loss at a particular frequency. The place code in the cochlea is “tonotopic”, each sound frequency stimulating a particular location best. Cochlear place is “tuned” to particular frequencies. But how is this frequency “tuning” established? Era of the “traveling wave” begins 1 kHz Georg von Békésy (1899-1972) Awarded a Nobel prize in 1961 for studies on cochlear mechanics. See “Experiments in hearing” compiled by Glen Weaver. “in situ” measurements • Improve visualization with silver grains and strobe illumination • Measure amp. of motion for constant input pressure at various frequencies. Tonotopic Distribution of Frequency The cochlea is a frequency analyzer, breaking complex waves into frequency components. Tonos = tone; Topia = place Tonotopic = frequency place code 4 kHz 1 kHz 250 Hz Passive mechanics largely due to gradual changes in the stiffness of the basilar membrane. Base: thick and narrow Apex: thin and wide A (Nonlinear) Amplifier in the Ear Gain = Amplitude of basilar membrane Amplitude of stapes motion Use laser to measure membrane at one tonotopic position. Compare with stapes motion to get gain. Vary frequency of sound stimulus while keeping input (stapes motion) constant. Passive mechanics gives the “dead or high level shape” (like with Békésy). Something else happens at low sound levels in an alive animal (like with psychoacoustics). -

The Underwater Piano: a Resonance Theory of Cochlear Mechanics
The Underwater Piano: A Resonance Theory of Cochlear Mechanics by James Andrew Bell A thesis submitted for the degree of Doctor of Philosophy of The Australian National University July 2005 Visual Sciences Group Research School of Biological Sciences The Australian National University Canberra, ACT 0200 Australia This thesis is my original work and has not been submitted, in whole or in part, for a degree at this or any other university. Nor does it contain, to the best of my knowledge and belief, any material published or written by any other person, except as acknowledged in the text. In particular, I acknowledge the contribution of Professor Neville H. Fletcher who wrote Appendix A of Bell & Fletcher (2004) [§R 5.6 in this thesis] and who helped refine the text of that paper. Dr Ted Maddess provided the draft Matlab code used to perform the autocorrelation analysis reported in Chapter R7. Sharyn Wragg, RSBS Illustrator, drew some of the figures as noted. Signed: ……………………………………………… Date: ………………………………………………… Acknowledgements This thesis would not have appeared without the encouragement, support, and interest of many people. Yet, among them, the primary mainstay of this enterprise has been my wife, Libby, who has stayed by me on this long journey. I thank her for understanding and tolerance. Rachel, Sarah, Micah, Adam, Daniel, and Joshua have given their love and support, too, and that has been a source of strength. My supervisors have contributed in large measure, and I am grateful to all of them. Professor Anthony W. Gummer of the University of Tübingen saw the potential of this work at an early stage, and I thank him for his initiative in opening the door to this PhD study and providing some support (a grant from the German Research Council, SFB 430, to A.W. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -
Cranial Nerve VIII
Cranial Nerve VIII Color Code Important (The Vestibulo-Cochlear Nerve) Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the students should be able to: ✓ List the nuclei related to vestibular and cochlear nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the vestibular pathways and its main connections. ✓ Describe the auditory pathway and its main connections. Due to the difference of arrangement of the lecture between the girls and boys slides we will stick to the girls slides then summarize the pathway according to the boys slides. Ponto-medullary Sulcus (cerebello- pontine angle) Recall: both cranial nerves 8 and 7 emerge from the ventral surface of the brainstem at the ponto- medullary sulcus (cerebello-pontine angle) Brain – Ventral Surface Vestibulo-Cochlear (VIII) 8th Cranial Nerve o Type: Special sensory (SSA) o Conveys impulses from inner ear to nervous system. o Components: • Vestibular part: conveys impulses associated with body posture ,balance and coordination of head & eye movements. • Cochlear part: conveys impulses associated with hearing. o Vestibular & cochlear parts leave the ventral surface* of brain stem through the pontomedullary sulcus ‘at cerebellopontine angle*’ (lateral to facial nerve), run laterally in posterior cranial fossa and enter the internal acoustic meatus along with 7th (facial) nerve. *see the previous slide Auditory Pathway Only on the girls’ slides 04:14 Characteristics: o It is a multisynaptic pathway o There are several locations between medulla and the thalamus where axons may synapse and not all the fibers behave in the same manner. -
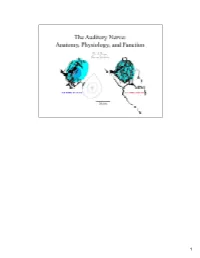
Auditory Nerve.Pdf
1 Sound waves from the auditory environment all combine in the ear canal to form a complex waveform. This waveform is deconstructed by the cochlea with respect to time, loudness, and frequency and neural signals representing these features are carried into the brain by the auditory nerve. It is thought that features of the sounds are processed centrally along parallel and hierarchical pathways where eventually percepts of the sounds are organized. 2 In mammals, the neural representation of acoustic information enters the brain by way of the auditory nerve. The auditory nerve terminates in the cochlear nucleus, and the cochlear nucleus in turn gives rise to multiple output projections that form separate but parallel limbs of the ascending auditory pathways. How the brain normally processes acoustic information will be heavily dependent upon the organization of auditory nerve input to the cochlear nucleus and on the nature of the different neural circuits that are established at this early stage. 3 This histology slide of a cat cochlea (right) illustrates the sensory receptors, the auditory nerve, and its target the cochlear nucleus. The orientation of the cut is illustrated by the pink line in the drawing of the cat head (left). We learned about the relationship between these structures by inserting a dye-filled micropipette into the auditory nerve and making small injections of the dye. After histological processing, stained single fibers were reconstruct back to their origin, and traced centrally to determine how they terminated in the brain. We will review the components of the nerve with respect to composition, innervation of the receptors, cell body morphology, myelination, and central terminations. -

A Rare Case of Hearing Impairment Due to Cerebello-Pontine Angle Lesion: Trigeminal Schwannoma
J Int Adv Otol 2015; 11(2): 170-2 • DOI: 10.5152/iao.2015.252 Case Report A Rare Case of Hearing Impairment due to Cerebello-Pontine Angle Lesion: Trigeminal Schwannoma Purushothaman Ganesan, Priya Sankaran, Purushothaman Pavanjur Kothandaraman SRM Medical College Hospital and Research Centre, Otorhinolaryngology, Audiology and Speech Language Pathology, Chennai, Tamilnadu, India Schwannoma of the trigeminal nerve is a rare condition. Even rarer is hearing loss occurring as a result of this lesion. The aim of this study is to highlight this rare cause of hearing impairment. Here we report the clinical features and findings of the imaging and audiological investigations of a case of trigeminal schwannoma diagnosed at our institution. Our patient presented with headache, giddiness, tinnitus, left-sided facial weakness, left-sided hearing loss, right-sided hemiplegia, and unintelligible speech. Radiological studies revealed a large well-defined mass lesion in the left cerebellopontine angle with a significant mass effect on posterior fossa structures, suggestive of trigeminal nerve tumor. Audio-vestibular assess- ment was done with pure tone audiometry, impedance audiometry, otoacoustic emission, brainstem-evoked response audiometry, and electronys- tagmography, which pointed toward a retrocochlear pathology for hearing loss and imbalance. KEYWORDS: Cerebellopontine angle tumor, vestibulocochlear nerve bundle, trigeminal schwannoma, electronystagmography INTRODUCTION Schwannomas, which usually exhibit benign behavior, are tumors arising from Schwann cells in the axon myelin sheaths [1]. Schwan- noma of the trigeminal nerve comprises only 0.2% to 0.4% of all intracranial tumors and primarily arises in the Gasserian ganglion [2]. They are relatively rare and less common than vestibular schwannoma [3]. They account for 0.07%–0.36% of all intracranial tumors and 0.8%–8% of intracranial schwannomas [4, 5]. -

Benzodiazepines Alter Cochleo-Cochlear Loop in Humans
Hearing Research 121 (1998) 71^76 Benzodiazepines alter cochleo-cochlear loop in humans N. Morand a, E. Veuillet a;*, M.C. Gagnieu b, P. Lemoine c, L. Collet a a Laboratoire `Neurosciences et Systeémes Sensoriels', 3 place d'Arsonval, Pavillon U, Hoêpital Edouard Herriot, 69003 Lyon, France b Laboratoire de Pharmacologie, Hoêpital Edouard Herriot, Lyon, France c Uniteè Clinique de Psychiatrie Biologique, Le Vinatier, Bron, France Received 26 November 1997; revised 26 March 1998; accepted 7 April 1998 Abstract By using otoacoustic emission, we looked for change in outer hair cell (OHC) motile activity and medial olivocochlear (MOC) system inhibition due to benzodiazepine administration, a drug that is known to produce a pharmacological effect by interacting with GABAergic inhibitory neurotransmission. No effect was observed on OHC motile activity, in contrast benzodiazepines decreased MOC system effectiveness suggesting the existence of GABAergic fibers projecting onto the MOC system. z 1998 Elsevier Science B.V. All rights reserved. Key words: Gamma-aminobutyric acid; Outer hair cell; Olivocochlear e¡erent system; Otoacoustic emission 1. Introduction OHCs, principally acetylcholine (Ach) and the inhibi- tory neurotransmitter Q-aminobutyric acid (GABA) (for The organ of Corti is innervated by the olivocochlear review see Eybalin, 1993). Concerning GABA, several e¡erent system (Rasmussen, 1946), in which two sub- studies seem to prove the presence of GABAergic syn- components have been clearly distinguished (Warr, apses in the cochlea. The presence of GABA and glu- 1992; Warr and Guinan, 1979). The lateral olivo- tamic acid decarboxylase in ¢bers and e¡erent endings cochlear (LOC) system originates from lateral superior synapsing onto OHC bodies has been revealed by im- olivary neurons making synaptic contact with the a¡er- munocytochemistry data (Fex and Altschuler, 1984; ent inner radial ¢bers of the inner hair cell system.