Factors Affecting Macadamia Nut Stability a Thesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An E-Recipe Book by Get Snacking 10 Tempting Macadamia Recipes
GET SNACKING AN E-RECIPE BOOK BY GET SNACKING 10 TEMPTING MACADAMIA RECIPES A mid-morning moment. The 3pm slump. A small treat after dinner. Whatever your time of choice, snacks are a small, but welcome, highlight of the day. The best snacks are those that are both tasty and nutritious, delivering the pleasure hit our brains crave, and the energy boost our bodies need. While busy lives can make it hard to stick to healthy habits, with just a little bit of preparation, it’s easy to stock up on winning snacks that everyone loves. As a nourishing wholefood, a handful of macadamias makes healthy snacking a breeze, and they’re an amazing addition to other snacks as well. This collection brings together 10 inspirational snacking ideas, so you’ll always have a tasty and nourishing snack ready to ward off pesky hunger pangs. We hope you enjoy exploring this new collection and feel inspired to make macadamias part of your everyday snacking routine. australian-macadamias.org MACADAMIA & VEGEMITE SCROLLS RECIPE OVER PAGE MACADAMIA Makes 10 500g plain bread flour plus ¼ cup for kneading and rolling & VEGEMITE 1 sachet dried yeast 1 teaspoon fine salt SCROLLS 325ml warm water 1 tablespoon olive oil These delicious scrolls feature the 2 tablespoons vegemite, or to taste classic Aussie combo of Vegemite 2 cups (125g) tasty cheese, grated and cheese but it’s the distinctive soft 3/4 cup raw macadamias, chopped roughly crunch of macadamias which really make them a guaranteed winner! Combine the flour, yeast and salt in a large bowl. -

List of Legumes
Healthy Oils & SmokePoints When it comes to the cooking oil in your cupboard, there is a huge difference in healthfulness depending on how the oil is stored and how it will be used. First let’s get some definitions for commonly used terms on labels and discussions about oils. Term Definition Cold Pressed Extracted without using heat. Extracted using a screw-type machine that presses the oil out. Can be done Expeller Pressed slowly, with very little heat, or can be done quickly with lots of friction and high temperatures. The first cold pressing which contains the best tasting and most healthful oils. Must contain less than 1% acids. By definition, this is cold-pressed and first Extra Virgin pressed, so don’t need to see these terms on the label. Must say 100% extra virgin, or may be a blend. The first cold pressing, but can contain a little more acids than the extra-virgin Virgin (1-3 percent). Seeds that have been genetically manipulated to decrease the amount of High Oleic essential fatty acids so that they have a longer shelf life. Are left in their state after pressing – no filtering. These oils tend to be more Unrefined Oils flavorful and richer in nutrients, however they have a very low smoke point. Oils have their impurities filtered out, to increase stability and allow for higher Refined temperature cooking. The processing can use toxic solvents, caustic soda, bleaches and phosphoric acid. Smoke Point Stage at which a heated oil begins to smoke, just before it bursts into flames. HEALTHY OILS & SMOKE POINTS PAGE | 1 © 2021 Health-Naturally.org Term Definition Oil should smell and taste like the food it came from. -

An E-Recipe Book by the Macadamia Beauty Book
AN E-RECIPE BOOK BY THE MACADAMIA BEAUTY BOOK 1 THE MACADAMIA BEAUTY BOOK As a lightweight, non-greasy moisturiser with proven anti-aging benefits, macadamia oil has become an essential ingredient in many luxury beauty products. But you don’t need to spend a fortune on day spa treatments to enjoy the benefits of this golden ingredient. Just a little of the oil applied daily can help your skin, hair and nails stay nourished and protected all year round. It’s also a 100% natural way to revitalise your skin. The recipes in our Macadamia Beauty Book showcase how easy it is to incorporate the simple luxury of macadamia oil into beauty products that you can make at home. Whether you need a face mask or a body balm, these recipes will nourish you from top to toe. Make sure you use a high-quality, cosmetic grade macadamia oil for these recipes rather than a culinary oil. It will have less impurities and a more neutral smell to leave you with that post-spa glow. It’s never been easier to experience the beauty benefits of macadamia oil. australian-macadamias.org 2 MACADAMIAS AND THEIR OIL CAN GIVE YOU THE BEAUTY BOOST YOU NEED, INSIDE AND OUT. Macadamias are a delicious way to add protein, calcium, potassium and dietary fibre to your diet, all of which we need to feel good on the inside – which helps us look great on the outside. Macadamias contain useful amounts of They are high manganese, an in palmitoleic antioxidant that is acid to help essential for your skin replenish your to produce collagen skins’ youthful to stay plump and radiance. -

Fats Ebook Feb 02.Pdf
2 DRHYMAN.COM Contents Contents INTRODUCTION ................................. 8 PART I ........................................... 11 Dietary Fats: The Good, Bad and the Ugly ............................................ 11 Fatty Acids ............................................................................................ 11 Saturated Fat ........................................................................................ 12 Polyunsaturated Fats ............................................................................ 14 Essential Fatty Acids 101- Omega-3 and Omega-6 ............................... 14 The Beneficial Omega-6 Fatty Acid: GLA ............................................... 16 How Fatty Acids Affect Brain Health ..................................................... 17 Omega-7 Fatty Acids ............................................................................ 18 Monounsaturated Fat ............................................................................ 18 Trans Fats ............................................................................................. 20 Trans Fats and Health ........................................................................... 21 Toxins in Fat .......................................................................................... 22 A Case for Organic ................................................................................ 23 DRHYMAN.COM 3 PART II .......................................... 24 Animal Fats ....................................................................... -

Specialty Oils Is Preventing That Remains After Pressing, and Virgin Olive Oxidation―Especially in Those That Contain a High Level of Oil
[Fats/Oils] Vol. 18 No. 9 September 2008 Pouring on Specialty Oil Ideas By Tamie Cook, Contributing Editor They say variety is the spice of life. And, if that’s the case, the product and menu developer’s pantry can be a veritable smorgasbord of piquancy. Literally, we have the world at the end of our whisk, and this has never been truer than when it comes to reaching for oils. In days past, choices were largely limited to vegetable, peanut, soybean, the occasional variety of olive oil and, on a good day, extra virgin olive oil. No longer confined to these basics, myriad choices now address today’s ingredient requirements for health, variety, taste and versatility. Whether it’s for a new dish on the menu or the next big product line, look at the choices, learn about their many uses and try some new oil on your palette. Most fruit, nut and seed oils generally undergo comparatively little processing. Many are mechanically, or “expeller,” extracted and are unrefined so no solvent was used to extract the oil, and no bleach or deodorizing agent was used to refine it. Thus, the oil retains its full, natural flavor, aroma and color, and many of its naturally occurring nutrients. This translates into a product that not only adds flavor to a dish, but also adds value to a menu or product. Shedding new light on an old friend When it comes to olive oil, everything old is new again. The olive tree, a native of Asia Minor, has been around for more than 6,000 years. -
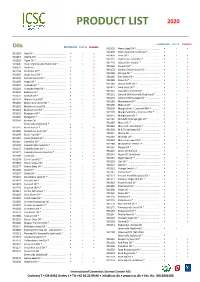
Product List 2020
PRODUCT LIST 2020 CONVENTIONAL ORGANIC STABILIZED Oils CONVENTIONAL ORGANIC STABILIZED 901199 Hemp Seed Oil * .................................. • • • 901499 Hemp Seed Oil Unrefined * ................. • • 901193 Acai Oil * ............................................. • • • 901450 Inchi Oil *............................................. • • • 901367 Alfalfa Oil* ........................................... • • • 901112 Jojoba Oil – Colorless * ........................ • • • 901228 Algae Oil * ........................................... • • • 901110 Jojoba Oil – Golden * ........................... • • • 907440 Aloe Oil (Internally Stabilized)* .......... • • 901162 Kakadu Oil * ........................................ • • • 906221 Amla Oil .............................................. • • 901152 Kalahari Melon Seed Oil * ................... • • • 901148 Andiroba Oil * ..................................... • • • 901168 Karanja Oil * ........................................ • • • 901387 Apple Seed Oil * .................................. • • • 901165 Kiwi Seed Oil * ..................................... • • • 901176 Apricot Kernel Oil * ............................. • • • 901185 Kukui Oil * ........................................... • • • 901195 Argan Oil * ........................................... • • • 901180 Lemon Seed Oil * ................................ • • • 901118 Avocado Oil * ...................................... • • • 901421 Lime Seed Oil * .................................... • • • 901218 Avocado Seed Oil * ............................. -
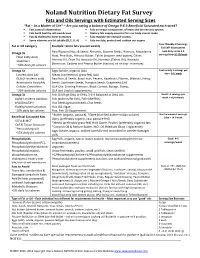
NN Fats and Oils with Serving Sizes
Noland Nutrition Dietary Fat Survey Fats and Oils Servings with Estimated Serving Sizes “Fat – As a Matter of Life” ~ Are you eating a balance of Omega 9-6-3-Beneficial Saturated each week? § Fats control inflammation § Fats are major components of brain and the nervous system § Fats build healthy cell membranes § Dietary fats supply essential fats our body cannot make § Fats & cholesterol form hormones § Fats regulate the immune system § Some vitamins are fat soluble (D, E, K, A) § Fats insulate, protect and cushion our organs Your Weekly # Servings Fat or Oil Category Example –circle fats you eat weekly Fat Self-Assessment Raw/Roasted Nuts & Seeds: Almonds, Sesame Seeds, Walnuts, Macadamia Goal daily intake 3-5 Omega 9s servings/day=20-30/week Oleic Fatty Acids Nuts, Pine Nuts, Almond Butter, Tahini (sesame seed butter), Olives Stabilizers Almond Oil, Olive Oil, Avocado Oil, Hummus (Tahini Oil), Avocado ~50% daily fat calories (Non-nuts: Cashew and Peanut Butter (natural, oil on top - minimize) Omega 6s Eggs (whole, organic) (AA) Goal variety 2 servings per Linoleic Acid (LA) Meats (commercial, grass-fed) (AA) day = 14 / week GLA (λ-linolenic acid) Raw Nuts & Seeds: Brazil nuts, Pecans, Hazelnuts, Filberts, Walnuts, Hemp Arachidonic Acid (AA) Seeds, Sunflower Seeds, Pumpkin Seeds, Grapeseed (LA) Cellular Controllers GLA-Oils: Evening Primrose, Black Currant, Borage, Hemp, ~30% daily fat calories GLA and Linoleic supplements Omega 3s Fish Oil (High DHA or EPA), 3-6-9 Balanced or DHA oils Goal 2 -3 servings per week + vit/minerals Alpha-Linolenic -

Yes, Our Newly Formulated Rejuvenating Shampoo Is Sulfate-Free, Paraben-Free and Color Safe. Yes, It Is Paraben-Free, Sulfate-Fr
FAQs Is The Rejuvenating Shampoo Sulfate Free? Yes, our newly formulated Rejuvenating Shampoo is sulfate-free, paraben-free and color safe. Is The Moisturizing Rinse Paraben-Free? Yes, it is paraben-free, sulfate-free and color safe. Are The Macadamia Nourishing Moisture Oil Treatment And Oil Spray Alcohol-Free? Yes, our amazing Oils are both indeed alcohol-free. Do Macadamia Professional Hair Products Contain Any Silicones? Silicones have been given quite the bad rap by those who overgeneralize, claiming negative effects such as build-up, drying and hair “suffocation”. In general, silicones serve many positive functions in hair care: reducing moisture loss, humidity-proofing, cuticle sealing and protection, detangling and shine enhancement. Some silicones are heavy and thick, some are quite water soluble and quickly evaporate; moreover, they can also be used in combination with each other and other ingredients to achieve a multitude of desirable effects. One of the things that make Macadamia Professional hair products stand apart is that we use a customized blend of high-quality silicones. These premium ingredients, when combined with our other signature ingredients, play an important role in producing the incredible results that our products are known for: Unprecedented softness, shine, manageability and protection with no greasiness or build-up. Are The Macadamia Natural Oil Products Ph Balanced? The Healing Oil Treatment and Deep Repair Masque have a pH of five, making them ideally balanced for your hair and skin’s natural pH. -

Saturated, Monounsaturated, and Polyunsaturated Fatty Acids Omega 6 and Omega 3
One of the most important parts of PALEO GUIDE TO OILS AND FATS eating Paleo is cutting out harmful oils that are all too common in the typical Western diet. There’s a lot that goes into what makes an oil or fat harmful or not, including whether its fatty acid composition is inflammatory and whether or not it is wrecked if heated too high. By the way, “fat” (lard, butter, etc.) is solid at room temperature and “oil” (corn oil, hazelnut oil, etc.) is liquid at room temperature. Here’s a primer on fats and oils. Use this guide to pick which ones you cook with, pour over salads, or avoid all together. First, all fats and oils are made up of a combination of fatty acids. None is completely saturated or unsaturated—not even lard—as you may have thought. In the upcoming charts, we’ve laid out the most common fats’ and oils’ fatty-acid concentrations, as well as the highest temperature we suggest you cook them at. SATURATED, MONOUNSATURATED, ANd POLYUNSATURATED FATTY ACIDS Polyunsaturated fatty acids (PUFAs), including omega 6 fatty acids (O6) and omega 3 fatty acids (O3), are delicate and easily oxidized by light, air, or heat. Oxidized fatty acids are what make an oil or fat rancid. Saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) are less susceptible to being oxidized and can stand up to more cooking heat than PUFAs can. OmEGA 6 AND OmEGA 3 While both O3s and O6s (remember, those are both PUFAs) are necessary nutrients for human health, O3s are anti-inflammatory, but O6s are inflammatory and can contribute to everything from heart disease to joint pain to skin problems. -

Lipid Profile and Antioxidant Activity of Macadamia Nuts (Macadamia Integrifolia) Cultivated in Venezuela
Natural Science, 2015, 7, 535-547 Published Online November 2015 in SciRes. http://www.scirp.org/journal/ns http://dx.doi.org/10.4236/ns.2015.712054 Lipid Profile and Antioxidant Activity of Macadamia Nuts (Macadamia integrifolia) Cultivated in Venezuela Alejandra Rengel1, Elevina Pérez1, George Piombo2, Julien Ricci3, Adrien Servent3, María Soledad Tapia1, Olivier Gibert3, Didier Montet3 1Instituto de Ciencia y Tecnología de Alimentos, Facultad de Ciencias, Universidad Central de Venezuela, Caracas, Venezuela 2Cirad, UMR Iate, Campus de la Gaillarde, 2 place Pierre Viala, Montpellier, France 3Cirad, UMR 95 Qualisud, TA B-95/16, 73, rue Jean-François Breton, Montpellier, France Received 19 July 2015; accepted 20 November 2015; published 24 November 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Macadamia nuts (Macadamia integrifolia) grown in Venezuela have showed an average total fat content of 70%. Oleic acid (18:1) was the main monounsaturated fatty acid (MUFA) (51.3%), followed by palmitoleic acid (16:1, 22.6%). The content of polyunsaturated fatty acids (PUFAs), C18:2 and C18:3 represented 5.4%. Thus, MUFAs and PUFAs together constituted more than 80% of the total fatty acids present. Trans-vaccenic acid was also present (3%). As regards to other phytochemical compounds, tocopherols and tocotrienols were not found in the sample, but the presence of squalene was detected. The antioxidant activity (44.2%) of the extract was produced by the phytochemicals present. Keywords Lipids, Antioxidant Activity, Fatty Acids, Macadamia Nut, Phytochemical Compounds 1. -

Macadamia Nut Oil Refined
MACADAMIA NUT OIL REFINED PRODUCT DATA SHEET MACADAMIA NUT OIL REFINED is a unique vegetable oil due to its high content in Palmitoleic acid, a monounsaturated fatty acid that avoids oxidation and fits with the skin s fatty acid composition. Palmitoleic acid is found in human sebum among the young, but the level dramatically drops in mature skin. MACADAMIA NUT OIL REFINED exhibits a long shelf life and good resistance to rancidity due to the low content of polyunsaturated fatty acids. It is an oil with minimal colour and virtually odourless. MACADAMIA NUT OIL REFINED applies easily and offers deep penetration and significant moisture retention together with high nourishing properties. Macadamia nut oil contains about 80% of monounsaturated fatty acids and a higher percentage of Palmitoleic acid than any other vegetable oil. MACADAMIA NUT OIL REFINED restores dry, dehydrated, and mature skin. MACADAMIA NUT OIL REFINED is suitable for all kinds of cosmetic products from rinse-off to leave-on. It is helpful in cases of sunburn and wound healing. TECHNICAL DATA Appearance: Clear pale yellow oily liquid Acidity index: 2.0 mg KOH/g oil Peroxide value: 10.0 meq O2/Kg oil Relative density (20ºC): 0.909 - 0.915 Fatty Acid Composition Stearic acid 2 - 5 % Palmitic acid 8 - 10 % Palmitoleic acid 16 - 24 Oleic acid 53 - 67 % Linoleic acid 1.5 - 4 % Updated: 04/2007 Approved: Dr. Blanca Martínez MACADAMIA NUT OIL REFINED MACADAMIA NUT OIL REFINED APPLICATION MACADAMIA NUT OIL REFINED maybedirectlyapplied to the skin and hair. It may also be easily incorporated as an active ingredient or an excellent carrier in skin and hair care products. -

High-Quality Fats
High-Quality Fats High-Quality Fats: Nuts/Seeds Foods High in MUFAs: Grass-Fed Beef o Almonds Olive oil Chia Seeds o Cashews Hazelnut oil Poultry: Organic o Almond Butter (unrefined) Wild Game o Macadamia Nuts Safflower oil Eggs o Pine Nuts (unrefined) Olives o Brazil Nuts Macadamia oil Avocados o Pecans Almond oil o Algae/Seaweed Hazelnuts/Filberts Tea seed oil o Sunflower Seeds Halibut Pecan oil o Pumpkin Seeds Shrimp Hazelnut oil Snapper (refined) Fats Better for Cooking: Coconut oil Healthiest Fats: Foods high in Omega 3s: Grass-Fed Butter Olive oil (virgin) Hemp oil Macadamia oil Hemp oil Flax oil/seeds/meal Almond oil (unrefined) Walnuts/Walnut oil Pecan oil Coconut oil Algae (unrefined) Hazelnut oil Chia Seeds Almond oil Avocado oil Salmon (unrefined/raw) Macadamia oil Scallops Tea Seed oil Tea Seed oil Soybeans Rice oil/Rice Bran Pecan oil Halibut oil Hazelnut oil (Baked/Broiled) (unrefined) Snapper (Baked) Fats Best for Avocado oil Tofu (raw) (unrefined) dressings/Sensitive to Winter Squash Flax oil (unrefined) high temperatures Tuna, yellowfin Olives (green or Safflower Cod (baked) black) (unrefined) Kidney beans Avocados Flax (unrefined) Hazelnut Other fats: (unrefined) Butter (grain fed) Olive Sesame Hemp (unrefined) Grapeseed Commonly Genetically Safflower (refined) Canola (Rapeseed) Modified Fats: Butter (Grass fed) (Conventional) oil Corn oil Ghee Cottonseed oil Margarine Palm and Palm Mayonnaise Kernel oil Shortening Soybean oil Hydrogenated oils HopeNaturalWellness.ca 403-382-8885 [email protected] .