X-Chromosome Inactivation and Skin Disease Bryan K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bbm:978-3-642-38765-4/1.Pdf
Index A Becker nevus and Becker nevus syndrome , Acantholysis, transient superfi cial , 158–159 14, 15, 86, 88–90 Acanthosis nigricans , 19, 20, 141–142, 145, 146 BFH. See Basaloid follicular hamartoma (BFH) Acne nevus of Munro , 91 Binary genodermatoses , 23, 115–118 Acne vulgaris , 26, 200–201, 203 aplasia cutis congenita Acrokeratoelastoidosis , 146 and nevus psiloliparus , 116, 117 AHO. See Albright’s hereditary osteodystrophy (AHO) and nevus sebaceous , 116 Aicardi syndrome , 188–189 nevus sebaceus and melorheostosis , 116 AKT1 mutation , 79, 83, 137 phacomatosis pigmentokeratotica , 115–116 Albinism-deafness syndrome, X-linked , 189–191 phacomatosis pigmentovascularis , 116–118 Albinism, X-linked oculocutaneous , 54 Birt-Hogg-Dubé syndrome. See Hornstein-Knickenberg Albright’s hereditary osteodystrophy (AHO) , 19, 20, syndrome 151–154 Blaschkitis , 49, 195, 205, 208 Allelic didymosis , 23–24, 109–111 Blaschko, Alfred , 9, 45, 47–51 capillary didymosis , 109–111 Blaschko dermatitis , 195 cutis tricolor , 110–111 Blaschko’s lines , 45–59, 63–64, 74 Darier disease , 111, 112 analogous patterns in other organs , 53–58 epidermolytic ichthyosis of Brocq , 111 in animals , 51, 54–56 Amelogenesis imperfecta , 53 atypical lines , 63–64 Anemic halo. See Rhodoid nevus brindle trait , 51, 54, 55 Angiokeratoma circumscriptum , 96 in broad bands , 46, 52–53 Angioma serpiginosum , 96–97 in chimeric mice , 51, 54 Angora hair nevus , 15, 86, 88–89 dwarf zebu, brindle trait , 54, 55 Apert syndrome , 91 embryonic cells , 49, 50 Archetypical patterns , 45–59 in epidermal nevi , 50, 52 Arteriovenous fi stulas , 20, 138, 156 on head and neck , 47, 50 Art nouveau , 57 intraoral lesions , 52 Atopic dermatitis , 26, 194, 195, 197–199 murine brain , 54, 56 ATP7A gene , 188 in narrow bands , 52 ATP2C1 mutation. -

ASDP 47Th Annual Meeting the American Society of Dermatopathology
ASDP 47th Annual Meeting The American Society of Dermatopathology October 7–10, 2010 Program Hilton Atlanta & Abstracts Atlanta, GA USA www.asdp.org The American Society of Dermatopathology The American Society of Dermatopathology Table of Contents General Information . 5 2010 Founders’ and Nickel Award Recipients . 7 Officers, Committees, Program Directors . 9 Past Presidents, Past Secretary Treasurers, Past Editors . 10 Committee Meetings . 12 Faculty Disclosures . 13 Schedules at a Glance Program at a Glance . 17 Consultations in Dermatopathology . 20 Schedule at a Glance . 20 Self-Assessment in Dermatopathology . 22 Schedule at a Glance . 22 Exhibits and Supporters Exhibit Floor Plan . 25 Exhibitors and Supporters . 25 Thursday, October 7 Daily Program . 31 Session Handouts . 37 Friday, October 8 Daily Program . 41 President’s Reception & Banquet . 46 Session Handouts . 47 Saturday, October 9 Daily Program . 51 Memorial Lecture . 54 Evening Slide Symposium Case Summaries . 57 Session Handouts . 59 Sunday, October 10 Daily Program . 63 Session Handouts . 67 Oral Abstracts . 71 Poster Abstracts . 95 Presenter Index . 195 ASDP 47th Annual Meeting The American Society of Dermatopathology ASDP 47th Annual Meeting 1 www.asdp.org The American Society of Dermatopathology The American Society of Dermatopathology • Differentiate essential diagnostic features that lead to the General Information accurate histologic assessment of surgical margins . Continuing Medical Education • Recognize light microscopic findings that lead to a diagnosis of The American Society of Dermatopathology is accredited by the specific inflammatory dermatoses . Accreditation Council for Continuing Medical Education to provide • Develop a diagnostic approach to the evaluation of biopsies continuing medical education for physicians .The American Soci- from inflammatory skin lesions . -

Laser Therapy for the Treatment of Morphea: a Systematic Review of Literature
Journal of Clinical Medicine Review Laser Therapy for the Treatment of Morphea: A Systematic Review of Literature Paulina Szczepanik-Kułak * , Małgorzata Michalska-Jakubus and Dorota Krasowska Chair and Department of Dermatology, Venerology and Paediatric Dermatology, Medical University of Lublin, 20-081 Lublin, Poland; [email protected] (M.M.-J.); [email protected] (D.K.) * Correspondence: [email protected] Abstract: Morphea, also known as localized scleroderma (LoS), comprises a set of autoimmune sclerotic skin diseases. It is characterized by inflammation and limited thickening and induration of the skin; however, in some cases, deeper tissues might also be involved. Although morphea is not considered a life-threatening disease, the apparent cosmetic disfigurement, functional or psychosocial impairment affects multiple fields of patients’ quality of life. Therapy for LoS is often unsatisfactory with numerous treatments that have only limited effectiveness or considerable side effects. Due to the advances in the application of lasers and their possible beneficial effects, the aim of this study is to review the reported usage of laser in morphea. We present a systematic review of available literature, performed with MEDLINE, Cinahl, Central, Scopus, Web of Science, and Google Scholar databases. We identified a total of twenty relevant studies (MEDLINE n = 10, Cinahl n = 1, Central n = 0, Scopus n = 2, Web of Science n = 5, Google Scholar n = 2) using laser therapy for LoS. Eight studies were focused on the use of PDL, six on fractional lasers (CO2 and Er:YAG), four on excimer, and two on either alexandrite or Nd:YAG. Keywords: morphea; localized scleroderma; laser therapy Citation: Szczepanik-Kułak, P.; Michalska-Jakubus, M.; Krasowska, D. -

The Classification of Capillary Malformations
The classification of capillary malformations Dubrovnik 3 May 2019 Rudolf Happle Department of Dermatology University of Freiburg Germany Background:: The unsuitable classification of the International Society for the Study of Vascular Anomalies (ISSVA) The unsuitable classification of the ISSVA Capillary malformation Capillary malformation Capillary malformation Capillary malformation Capillary malformation Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies Wassef M et al and ISSVA Board and Scientific Committee: Pediatrics 2015 Klippel-Trenaunay syndrome: CM + VM +/− LM + limb overgrowth Parkes-Weber syndrome: CM + AVF + limb overgrowth Servelle-Martorell syndrome: limb VM + bone undergrowth Sturge-Weber syndrome: facial + leptomeningeal CM + ocular anomalies +/− bone and/or soft tissue overgrowth Limb CM + congenital nonprogressive limb hypertrophy Maffucci syndrome: VM +/− spindle cell hemangioma + enchondroma Macrocephaly-CM (M-CM)/megalencephaly-CM-polymicrogyria (MCAP) Microcephaly-CM (MICCAP) CLOVES syndrome: LM + VM + CM +/− AVM + lipomatous overgrowth Proteus syndrome: CM, VM and/or LM + asymmetric somatic overgrowth Bannayan-Riley-Ruvalcaba syndrome: AVM + VM + macrocephaly, lipomatous overgrowth Approved in Amsterdam, May 2018 For pediatric dermatologists, this classification is useless! http://www.issva.org/classification All of these capillary nevi may occur without any extracutaneous abnormality! Nevus flammeus Nevus roseus Rhodoid nevus Nevus vascularis -

Mallory Prelims 27/1/05 1:16 Pm Page I
Mallory Prelims 27/1/05 1:16 pm Page i Illustrated Manual of Pediatric Dermatology Mallory Prelims 27/1/05 1:16 pm Page ii Mallory Prelims 27/1/05 1:16 pm Page iii Illustrated Manual of Pediatric Dermatology Diagnosis and Management Susan Bayliss Mallory MD Professor of Internal Medicine/Division of Dermatology and Department of Pediatrics Washington University School of Medicine Director, Pediatric Dermatology St. Louis Children’s Hospital St. Louis, Missouri, USA Alanna Bree MD St. Louis University Director, Pediatric Dermatology Cardinal Glennon Children’s Hospital St. Louis, Missouri, USA Peggy Chern MD Department of Internal Medicine/Division of Dermatology and Department of Pediatrics Washington University School of Medicine St. Louis, Missouri, USA Mallory Prelims 27/1/05 1:16 pm Page iv © 2005 Taylor & Francis, an imprint of the Taylor & Francis Group First published in the United Kingdom in 2005 by Taylor & Francis, an imprint of the Taylor & Francis Group, 2 Park Square, Milton Park Abingdon, Oxon OX14 4RN, UK Tel: +44 (0) 20 7017 6000 Fax: +44 (0) 20 7017 6699 Website: www.tandf.co.uk All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of the publisher or in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of any licence permitting limited copying issued by the Copyright Licensing Agency, 90 Tottenham Court Road, London W1P 0LP. Although every effort has been made to ensure that all owners of copyright material have been acknowledged in this publication, we would be glad to acknowledge in subsequent reprints or editions any omissions brought to our attention. -
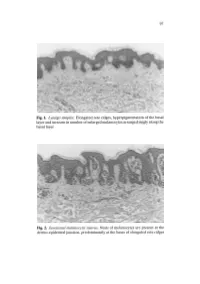
Fig. 1. Lentigo Simplex. Elongated Rete Ridges, Hyperpigmentation of The
97 Fig. 1. Lentigo simplex. Elongated rete ridges, hyperpigmentation of the basal layer and increase in number of enlarged melanocytes arranged singly along the basal layer Fig. 2. Junctional melanocytic naevus. Nests of melanocytes are present at the dermo-epidermal junction, predominantly at the bases of elongated rete ridges 98 a b Fig.3a,b. Compound melanocytic naevus. a Nests of melanocytes in the dermis as well as the derma-epidermal junction, smaller and more diffusely arranged in the deeper dermis. b The nests of melanocytes include multinucleated giant cells 99 Fig. 4. Dermal melanocytic naevus. Entirely intradermal tumour in a nested pattern in the superficial layers, composed of small naevoid melanocytes in the deep dermis Fig. 5. Dermal melanocytic naevus. The deeper layers of this dermal melanocytic naevus include neuroid structures similar to Wagner-Meissner corpuscles (neuroid dermal melanocytic naevus) 100 Fig. 6. Balloon cell naevus. The tumour is composed mainly of melanocytes with abundant clear cytoplasm and central nuclei Fig. 7. Halo naevus. Compound naevus with a dense lymphocytic infiltrate between the naevoid melanocytes of the dermal component Fig. 8. Combined intradermal naevus and blue naevus. Nests of epithelioid naevus cells in the dermis (right) and heavily pigmented spindle and dendritic cells arranged between collagen bundles in the adjacent stroma (left) Fig. 9a. Deep penetrating naevus. A wedge-shaped lesion composed of irregu lar collections of variably pigmented cells extending into the deep dermis. 102 Fig. 9b. Deep penetrating naevus. In the deep dermis the lesion includes large cells with nuclear pseudo-inclusions, smaller naevoid melanocytes and groups of melanophages in the intervening stroma Fig. -

Pigmented Purpuric Dermatosis: a Review of the Literatureଝ
Actas Dermosifiliogr. 2020;111(3):196---204 REVIEW Pigmented Purpuric Dermatosis: A Review of the Literatureଝ ∗ I. Martínez Pallás, R. Conejero del Mazo, V. Lezcano Biosca Servicio de Dermatología y Venereología, Hospital Clínico Lozano Blesa, Zaragoza, Spain Received 21 October 2018; accepted 24 February 2019 Available online 20 March 2020 KEYWORDS Abstract The pigmented purpuric dermatoses (PPDs) are a group of benign, chronic diseases. The variants described to date represent different clinical presentations of the same entity, Purpuric pigmented dermatosis; all having similar histopathologic characteristics. We provide an overview of the most common Review; PPDs and describe their clinical, dermatopathologic, and epiluminescence features. PPDs are both rare and benign, and this, together with an as yet poor understanding of the pathogenic Clinical presentation; Treatment mechanisms involved, means that no standardized treatments exist. We review the treatments described to date. However, because most of the descriptions are based on isolated cases or small series, there is insufficient evidence to support the use of any of these treatments as first-line therapy. © 2019 AEDV. Published by Elsevier Espana,˜ S.L.U. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). PALABRAS CLAVE Dermatosis purpúricas pigmentadas. Revisión de la literatura científica Dermatosis purpúrico pigmentadas; Resumen Las dermatosis purpúrico pigmentadas (DPP) son un grupo de enfermedades benig- Revisión; nas y de curso crónico. Las variantes descritas representan distintas formas clínicas de una misma entidad con unas características histopatológicas comunes para todas ellas. Exponemos Presentación clínica; Tratamiento a continuación un resumen de las variedades más frecuentes, sus características clínicas, der- matopatológicas y de epiluminiscencia. -

Dermatology: Practical and Conceptual
DERMATOLOGY PRACTICAL & CONCEPTUAL www.derm101.com Abstracts from the 4th World Congress of the International Dermoscopy Society, April 16-18, 2015, Vienna, Austria Citation: Abstracts from the 4th World Congress of the International Dermoscopy Society, April 16-18, 2015, Vienna, Austria. Dermatol Pract Concept 2015;5(2):28. http://dx.doi.org/10.5826/dpc.0502a28 ORAL PRESENTATIONS carcinoma and basal cell carcinoma), as well as infectious and genetic disease (storage diseases), could be evocated. The detection of parasites (Demodex folliculorum) on eye- FREE COMMUNICATIONS: lids was possible. Confocal images correlated well with con- CONFOCAL AND OCT ventional histopathology. The fluorescence examination of corneal squamous cell carcinoma by VivaScope 1500 was FC1-1 characterized by extravasation of fluorescein after intrave- nous injection. IN VIVO CONFOCAL MICROSCOPES DEDICATED TO THE SKIN FOR THE EXPLORATION OF THE Conclusions: Confocal microscopes dedicated to the skin of- fer new perspectives for the diagnosis, optimization of treat- OCULAR SURFACE: A NEW PERSPECTIVE FOR ments, and follow-up of the ocular diseases. They will allow DERMATOLOGISTS dermatologists to examine conjunctival and eyelid tumors, as it is for skin or genital mucosa. In addition, thanks to *1 1 1 Elisa Cinotti , Jean-Luc Perrot , Bruno Labeille , some adaptations of the dermatological device VivaScope® Gilles Thuret2, Damien Grivet2, Philippe Gain2, 1500, it is possible for the first time to perform a fluorsecnte Frédéric Cambazard1 examination of the ocular and peri-ocular tissue, opening a new era in the clinical imaging of the ocular surface. A new 1 2 Dermatology, Opthalmology, University Hospital of Saint-Etienne, semiology remains to be learned. -

Read Code Description 14L.. H/O: Drug Allergy 158.. H/O: Abnormal Uterine Bleeding 16C2
Read Code Description 14L.. H/O: drug allergy 158.. H/O: abnormal uterine bleeding 16C2. Backache 191.. Tooth symptoms 191Z. Tooth symptom NOS 1927. Dry mouth 198.. Nausea 199.. Vomiting 19C.. Constipation 1A23. Incontinence of urine 1A32. Cannot pass urine - retention 1B1G. Headache 1B62. Syncope/vasovagal faint 1B75. Loss of vision 1BA2. Generalised headache 1BA3. Unilateral headache 1BA4. Bilateral headache 1BA5. Frontal headache 1BA6. Occipital headache 1BA7. Parietal headache 1BA8. Temporal headache 1C13. Deafness 1C131 Unilateral deafness 1C132 Partial deafness 1C133 Bilateral deafness 1C14. "Blocked ear" 1C15. Popping sensation in ear 1C1Z. Hearing symptom NOS 22J.. O/E - dead 22J4. O/E - dead - sudden death 22L4. O/E - Wound infected 2542. O/E - dental caries 2554. O/E - gums - blue line 2555. O/E - hypertrophy of gums 2FF.. O/E - skin ulcer 2I14. O/E - a rash 39C0. Pressure sore 39C1. Superficial pressure sore 39C2. Deep pressure sore 62... Patient pregnant 6332. Single stillbirth 66G4. Allergy drug side effect 72001 Enucleation of eyeball 7443. Exteriorisation of trachea 744D. Tracheo-oesophageal puncture 7511. Surgical removal of tooth 75141 Root canal therapy to tooth 7610. Total excision of stomach 7645. Creation of ileostomy 773C. Other operations on bowel 773Cz Other operation on bowel NOS 7826. Incision of bile duct 7840. Total excision of spleen 7B01. Total nephrectomy 7C032 Unilateral total orchidectomy - unspecified 7E117 Left salpingoophorectomy 7E118 Right salpingectomy 7E119 Left salpingectomy 7G321 Avulsion of nail 7H220 Exploratory laparotomy 7J174 Manipulation of mandible 8HG.. Died in hospital 94B.. Cause of death A.... Infectious and parasitic diseases A0... Intestinal infectious diseases A00.. Cholera A000. -
Use of Serine Protease Inhibitors in the Treatment
(19) TZZ _T (11) EP 2 244 728 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61P 17/00 (2006.01) A61K 38/55 (2006.01) 31.08.2016 Bulletin 2016/35 A61Q 19/00 (2006.01) A61K 38/48 (2006.01) G01N 33/50 (2006.01) (21) Application number: 09703640.4 (86) International application number: (22) Date of filing: 21.01.2009 PCT/IB2009/000089 (87) International publication number: WO 2009/093119 (30.07.2009 Gazette 2009/31) (54) USE OF SERINE PROTEASE INHIBITORS IN THE TREATMENT OF SKIN DISEASES VERWENDUNG VON SERINPROTEASEHEMMERN BEI DER BEHANDLUNG VON HAUTERKRANKUNGEN UTILISATION D’INHIBITEURSDE SÉRINE PROTÉASES POUR TRAITER DES MALADIES DE PEAU (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR EP-A- 0 567 816 WO-A-00/07620 HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL WO-A-2004/045634 WO-A-2005/117955 PT RO SE SI SK TR WO-A-2006/037606 WO-A-2006/090282 WO-A-2007/072012 WO-A-2009/024528 (30) Priority: 21.01.2008 US 22386 US-A1- 2003 054 445 22.01.2008 US 6576 • ONG C ET AL: "LEKTI demonstrable by (43) Date of publication of application: immunohistochemistry of the skin: a potential 03.11.2010 Bulletin 2010/44 diagnostic skin test for Netherton syndrome" BRITISHJOURNAL OF DERMATOLOGY,vol. 151, (73) Proprietors: no.6, December 2004 (2004-12), pages 1253-1257, • DERMADIS XP002548095 ISSN: 0007-0963 74160 Archamps (FR) • SCHECHTER N M ET AL: "Inhibition of human • Institut National de la Santé et de la Recherche kallikrein-5 (SCTE) and-7 (SCCE) by Médicale lympho-epithelial Kazal-type inhibitor (LEKTI) 75013 Paris (FR) supports a role for proteolysis in Netherton disease and desquamation" JOURNAL OF (72) Inventors: INVESTIGATIVE DERMATOLOGY, vol. -
A Clinical Study on Genodermatoses and Their Effect On
A CLINICAL STUDY ON GENODERMATOSES AND THEIR EFFECT ON QUALITY OF LIFE – PROSPECTIVE OBSERVATIONAL STUDY IN A TERTIARY CARE HOSPITAL Dissertation Submitted to THE TAMILNADU DR.M.G.R. MEDICAL UNIVERSITY IN PARTIAL FULFILMENT FOR THE AWARD OF THE DEGREE OF DOCTOR OF MEDICINE IN DERMATOLOGY, VENEREOLOGY & LEPROSY Register No.: 201730253 BRANCH XX MAY 2020 DEPARTMENT OF DERMATOLOGY VENEREOLOGY & LEPROSY TIRUNELVELI MEDICAL COLLEGE TIRUNELVELI -11 BONAFIDE CERTIFICATE This is to certify that the dissertation titled as “A CLINICAL STUDY ON GENODERMATOSES AND THEIR EFFECT ON QUALITY OF LIFE – PROSPECTIVE OBSERVATIONAL STUDY IN A TERTIARY CARE HOSPITAL” submitted by Dr.P.KARTHIKRAJA to the Tamil Nadu Dr.M.G.R. Medical University, Chennai,in partial fulfilment of the requirement for the award of the Degree of DOCTOR OF MEDICINE in DERMATOLOGY, VENEREOLOGY AND LEPROSY during the academic period 2017 – 2020 is a bonafide research work carried out by him under direct supervision & guidance. Dr.P.Nirmaladevi MD., Dr.S.M.Kannan MS , MCh., Professor and HOD The Dean Department of Dermatology,Venereology &Leprosy Tirunelveli Medical College Tirunelveli Medical College Tirunelveli Tirunelveli CERTIFICATE This is to certify that the dissertation titled as “A CLINICAL STUDY ON GENODERMATOSES AND THEIR EFFECT ON QUALITY OF LIFE – PROSPECTIVE OBSERVATIONAL STUDY IN A TERTIARY CARE HOSPITAL” submitted by Dr.P.KARTHIKRAJA is a original work done by him in the Department of Dermatology,Venereology & Leprosy,Tirunelveli Medical College,Tirunelveli for the award -

Extensive Pigmented Purpuric Dermatosis Successfully Treated with Pentoxifylline Ann Dermatol Vol
Extensive Pigmented Purpuric Dermatosis Successfully Treated with Pentoxifylline Ann Dermatol Vol. 24, No. 3, 2012 http://dx.doi.org/10.5021/ad.2012.24.3.363 LETTER TO THE EDITOR Extensive Pigmented Purpuric Dermatosis Successfully Treated with Pentoxifylline Je-Ho Mun, M.D., Seung-Wook Jwa, M.D., Margaret Song, M.D., Hoon-Soo Kim, M.D., Hyun-Chang Ko, M.D., Byung-Soo Kim, M.D., Moon-Bum Kim, M.D. Department of Dermatology, Pusan National University Hospital, Pusan National University School of Medicine, Medical Research Institute, Busan, Korea Dear editor: orange-brown background (Fig. 1d). No abnormalities Pigmented purpuric dermatoses (PPDs) are a group of were found on routine laboratory tests. A punch biopsy chronic and relapsing dermatoses which are morpho- sample obtained from the left arm showed mild epidermal logically different but histopathologically indistinguishable1. hyperkeratosis, orthokeratosis, and perivascular lymphocyte They are characterized by petechiae, pigmentation, and and histocyte infiltration in the papillary dermis with focal occasionally, telangiectasia, and typically localized to the extravasations of erythrocytes (Fig. 1e), suggesting PPD. lower limbs. The cause of PPDs is largely unknown. These The patient was treated with pentoxifylline 400 mg bid for dermatoses are histopathologically similar to perivascular 1 month. In a follow-up visit, she showed significant lymphocyte infiltration, erythrocyte extravasation, and improvement of the lesion. hemosiderin deposition. Although clinical subclassification A 20-year-old female patient (case 2) had a 2-year history attempts to account for clinical variations between PPDs, of red-brown hyperpigmented patches in a band-like overlapping categories may make differentiation challenging. pattern on the arms and legs without subjective symptoms.