The Skull Roof Tracks the Brain During the Evolution and Development of Reptiles Including Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

8. Archosaur Phylogeny and the Relationships of the Crocodylia
8. Archosaur phylogeny and the relationships of the Crocodylia MICHAEL J. BENTON Department of Geology, The Queen's University of Belfast, Belfast, UK JAMES M. CLARK* Department of Anatomy, University of Chicago, Chicago, Illinois, USA Abstract The Archosauria include the living crocodilians and birds, as well as the fossil dinosaurs, pterosaurs, and basal 'thecodontians'. Cladograms of the basal archosaurs and of the crocodylomorphs are given in this paper. There are three primitive archosaur groups, the Proterosuchidae, the Erythrosuchidae, and the Proterochampsidae, which fall outside the crown-group (crocodilian line plus bird line), and these have been defined as plesions to a restricted Archosauria by Gauthier. The Early Triassic Euparkeria may also fall outside this crown-group, or it may lie on the bird line. The crown-group of archosaurs divides into the Ornithosuchia (the 'bird line': Orn- ithosuchidae, Lagosuchidae, Pterosauria, Dinosauria) and the Croco- dylotarsi nov. (the 'crocodilian line': Phytosauridae, Crocodylo- morpha, Stagonolepididae, Rauisuchidae, and Poposauridae). The latter three families may form a clade (Pseudosuchia s.str.), or the Poposauridae may pair off with Crocodylomorpha. The Crocodylomorpha includes all crocodilians, as well as crocodi- lian-like Triassic and Jurassic terrestrial forms. The Crocodyliformes include the traditional 'Protosuchia', 'Mesosuchia', and Eusuchia, and they are defined by a large number of synapomorphies, particularly of the braincase and occipital regions. The 'protosuchians' (mainly Early *Present address: Department of Zoology, Storer Hall, University of California, Davis, Cali- fornia, USA. The Phylogeny and Classification of the Tetrapods, Volume 1: Amphibians, Reptiles, Birds (ed. M.J. Benton), Systematics Association Special Volume 35A . pp. 295-338. Clarendon Press, Oxford, 1988. -

New Permian Fauna from Tropical Gondwana
ARTICLE Received 18 Jun 2015 | Accepted 18 Sep 2015 | Published 5 Nov 2015 DOI: 10.1038/ncomms9676 OPEN New Permian fauna from tropical Gondwana Juan C. Cisneros1,2, Claudia Marsicano3, Kenneth D. Angielczyk4, Roger M. H. Smith5,6, Martha Richter7, Jo¨rg Fro¨bisch8,9, Christian F. Kammerer8 & Rudyard W. Sadleir4,10 Terrestrial vertebrates are first known to colonize high-latitude regions during the middle Permian (Guadalupian) about 270 million years ago, following the Pennsylvanian Gondwanan continental glaciation. However, despite over 150 years of study in these areas, the bio- geographic origins of these rich communities of land-dwelling vertebrates remain obscure. Here we report on a new early Permian continental tetrapod fauna from South America in tropical Western Gondwana that sheds new light on patterns of tetrapod distribution. Northeastern Brazil hosted an extensive lacustrine system inhabited by a unique community of temnospondyl amphibians and reptiles that considerably expand the known temporal and geographic ranges of key subgroups. Our findings demonstrate that tetrapod groups common in later Permian and Triassic temperate communities were already present in tropical Gondwana by the early Permian (Cisuralian). This new fauna constitutes a new biogeographic province with North American affinities and clearly demonstrates that tetrapod dispersal into Gondwana was already underway at the beginning of the Permian. 1 Centro de Cieˆncias da Natureza, Universidade Federal do Piauı´, 64049-550 Teresina, Brazil. 2 Programa de Po´s-Graduac¸a˜o em Geocieˆncias, Departamento de Geologia, Universidade Federal de Pernambuco, 50740-533 Recife, Brazil. 3 Departamento de Cs. Geologicas, FCEN, Universidad de Buenos Aires, IDEAN- CONICET, C1428EHA Ciudad Auto´noma de Buenos Aires, Argentina. -

Il/I,E,Icanjluseum
il/i,e,icanJluseum PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK 24, N.Y. NUMBER 1870 FEBRUARY 26, 1958 The Role of the "Third Eye" in Reptilian Behavior BY ROBERT C. STEBBINS1 AND RICHARD M. EAKIN2 INTRODUCTION The pineal gland remains an organ of uncertain function despite extensive research (see summaries of literature: Pflugfelder, 1957; Kitay and Altschule, 1954; and Engel and Bergmann, 1952). Its study by means of pinealectomy has been hampered in the higher vertebrates by its recessed location and association with large blood vessels which have made difficult its removal without brain injury or serious hemorrhage. Lack of purified, standardized extracts, improper or inadequate extrac- tion techniques (Quay, 1956b), and lack of suitable assay methods to test biological activity have hindered the physiological approach. It seems probable that the activity of the gland varies among different species (Engel and Bergmann, 1952), between individuals of the same species, and within the same individual. This may also have contrib- uted to the variable results obtained with pinealectomy, injection, and implantation experiments. The morphology of the pineal apparatus is discussed in detail by Tilney and Warren (1919) and Gladstone and Wakely (1940). Only a brief survey is presented here for orientation. In living vertebrates the pineal system in its most complete form may be regarded as consisting of a series of outgrowths situated above the third ventricle in the roof of the diencephalon. In sequence these outgrowths are the paraphysis, dorsal sac, parapineal, and pineal bodies. The paraphysis, the most I University of California Museum of Vertebrate Zoology. -

Early Tetrapod Relationships Revisited
Biol. Rev. (2003), 78, pp. 251–345. f Cambridge Philosophical Society 251 DOI: 10.1017/S1464793102006103 Printed in the United Kingdom Early tetrapod relationships revisited MARCELLO RUTA1*, MICHAEL I. COATES1 and DONALD L. J. QUICKE2 1 The Department of Organismal Biology and Anatomy, The University of Chicago, 1027 East 57th Street, Chicago, IL 60637-1508, USA ([email protected]; [email protected]) 2 Department of Biology, Imperial College at Silwood Park, Ascot, Berkshire SL57PY, UK and Department of Entomology, The Natural History Museum, Cromwell Road, London SW75BD, UK ([email protected]) (Received 29 November 2001; revised 28 August 2002; accepted 2 September 2002) ABSTRACT In an attempt to investigate differences between the most widely discussed hypotheses of early tetrapod relation- ships, we assembled a new data matrix including 90 taxa coded for 319 cranial and postcranial characters. We have incorporated, where possible, original observations of numerous taxa spread throughout the major tetrapod clades. A stem-based (total-group) definition of Tetrapoda is preferred over apomorphy- and node-based (crown-group) definitions. This definition is operational, since it is based on a formal character analysis. A PAUP* search using a recently implemented version of the parsimony ratchet method yields 64 shortest trees. Differ- ences between these trees concern: (1) the internal relationships of aı¨stopods, the three selected species of which form a trichotomy; (2) the internal relationships of embolomeres, with Archeria -

A Small Lepidosauromorph Reptile from the Early Triassic of Poland
A SMALL LEPIDOSAUROMORPH REPTILE FROM THE EARLY TRIASSIC OF POLAND SUSAN E. EVANS and MAGDALENA BORSUK−BIAŁYNICKA Evans, S.E. and Borsuk−Białynicka, M. 2009. A small lepidosauromorph reptile from the Early Triassic of Poland. Palaeontologia Polonica 65, 179–202. The Early Triassic karst deposits of Czatkowice quarry near Kraków, southern Poland, has yielded a diversity of fish, amphibians and small reptiles. Two of these reptiles are lepido− sauromorphs, a group otherwise very poorly represented in the Triassic record. The smaller of them, Sophineta cracoviensis gen. et sp. n., is described here. In Sophineta the unspecial− ised vertebral column is associated with the fairly derived skull structure, including the tall facial process of the maxilla, reduced lacrimal, and pleurodonty, that all resemble those of early crown−group lepidosaurs rather then stem−taxa. Cladistic analysis places this new ge− nus as the sister group of Lepidosauria, displacing the relictual Middle Jurassic genus Marmoretta and bringing the origins of Lepidosauria closer to a realistic time frame. Key words: Reptilia, Lepidosauria, Triassic, phylogeny, Czatkowice, Poland. Susan E. Evans [[email protected]], Department of Cell and Developmental Biology, Uni− versity College London, Gower Street, London, WC1E 6BT, UK. Magdalena Borsuk−Białynicka [[email protected]], Institut Paleobiologii PAN, Twarda 51/55, PL−00−818 Warszawa, Poland. Received 8 March 2006, accepted 9 January 2007 180 SUSAN E. EVANS and MAGDALENA BORSUK−BIAŁYNICKA INTRODUCTION Amongst living reptiles, lepidosaurs (snakes, lizards, amphisbaenians, and tuatara) form the largest and most successful group with more than 7 000 widely distributed species. The two main lepidosaurian clades are Rhynchocephalia (the living Sphenodon and its extinct relatives) and Squamata (lizards, snakes and amphisbaenians). -

PATHOLOGICAL CRANIAL LESIONS in a JUVENILE CRANIAL COLLECTION a Thesis Submitted to the Faculty of San Francisco State Universit
PATHOLOGICAL CRANIAL LESIONS IN A JUVENILE CRANIAL COLLECTION A Thesis submitted to the faculty of San Francisco State University in partial fulfillment of A - the Requirements for -i r the degree M2 Master of Arts In . IsA 53 Anthropology by Hannah Marie Miller San Francisco, California January 2018 Copyright By Hannah Marie Miller 2018 CERTIFICATION OF APPROVAL I certify that I have read Pathological Cranial Lesions in a Juvenile Cranial Collection by Hannah Marie Miller, and that in my opinion this work meets the criteria for approving a thesis submitted in partial fulfillment of the requests for the degree: Master of Arts in Anthropology at San Francisco State University. Cymhia Wilczak Associate Professor of Anthropology Associate Professor of Anthropology PATHOLOGICAL CRANIAL LESIONS IN A JUVENILE CRANIAL COLLECTION Hannah Marie Miller San Francisco, California 2018 The purpose of this study is to examine and analyze the age distributions and co-occurrence of endocranial and ectocranial lesions commonly associated with metabolic disease and inflammatory processes in juveniles. The pathogenic changes studied are porotic hyperostosis, cribra orbitalia and endocranial lesions. The specific etiology of any of these lesions remains uncertain. (Lewis 2004, Janovic et al. 2012, Walker et al. 2009, Wilczak and Zimova Hopkins 2010). While porotic hyperostosis and cribra orbitalia have been subject to multiple studies, to date no one has statistically confirmed a relationship between these lesions. Endocranial lesions have never been systematically studied in conjunction with porotic hyperostosis or cribra orbitalia. As Wilczak and Zimova Hopkins (2010) suggested that not all lesions classified as cribra orbitalia shared a common etiology, classification of porotic hyperostosis and cribra orbitalia were modified for analysis here. -

Anatomy and Relationships of the Triassic Temnospondyl Sclerothorax
Anatomy and relationships of the Triassic temnospondyl Sclerothorax RAINER R. SCHOCH, MICHAEL FASTNACHT, JÜRGEN FICHTER, and THOMAS KELLER Schoch, R.R., Fastnacht, M., Fichter, J., and Keller, T. 2007. Anatomy and relationships of the Triassic temnospondyl Sclerothorax. Acta Palaeontologica Polonica 52 (1): 117–136. Recently, new material of the peculiar tetrapod Sclerothorax hypselonotus from the Middle Buntsandstein (Olenekian) of north−central Germany has emerged that reveals the anatomy of the skull and anterior postcranial skeleton in detail. Despite differences in preservation, all previous plus the new finds of Sclerothorax are identified as belonging to the same taxon. Sclerothorax is characterized by various autapomorphies (subquadrangular skull being widest in snout region, ex− treme height of thoracal neural spines in mid−trunk region, rhomboidal interclavicle longer than skull). Despite its pecu− liar skull roof, the palate and mandible are consistent with those of capitosauroid stereospondyls in the presence of large muscular pockets on the basal plate, a flattened edentulous parasphenoid, a long basicranial suture, a large hamate process in the mandible, and a falciform crest in the occipital part of the cheek. In order to elucidate the phylogenetic position of Sclerothorax, we performed a cladistic analysis of 18 taxa and 70 characters from all parts of the skeleton. According to our results, Sclerothorax is nested well within the higher stereospondyls, forming the sister taxon of capitosauroids. Palaeobiologically, Sclerothorax is interesting for its several characters believed to correlate with a terrestrial life, although this is contrasted by the possession of well−established lateral line sulci. Key words: Sclerothorax, Temnospondyli, Stereospondyli, Buntsandstein, Triassic, Germany. -

Resolving Homology in the Face of Shifting Germ Layer Origins
REVIEW ARTICLE Resolving homology in the face of shifting germ layer origins: Lessons from a major skull vault boundary Camilla S Teng1,2†, Lionel Cavin3, Robert E Maxson Jnr2, Marcelo R Sa´ nchez-Villagra4, J Gage Crump1* 1Department of Stem Cell Biology and Regenerative Medicine, University of Southern California, Los Angeles, United States; 2Department of Biochemistry, Keck School of Medicine, University of Southern California, Los Angeles, United States; 3Department of Earth Sciences, Natural History Museum of Geneva, Geneva, Switzerland; 4Paleontological Institute and Museum, University of Zurich, Zurich, Switzerland Abstract The vertebrate skull varies widely in shape, accommodating diverse strategies of feeding and predation. The braincase is composed of several flat bones that meet at flexible joints called sutures. Nearly all vertebrates have a prominent ‘coronal’ suture that separates the front and back of the skull. This suture can develop entirely within mesoderm-derived tissue, neural crest- derived tissue, or at the boundary of the two. Recent paleontological findings and genetic insights in non-mammalian model organisms serve to revise fundamental knowledge on the development and evolution of this suture. Growing evidence supports a decoupling of the germ layer origins of *For correspondence: the mesenchyme that forms the calvarial bones from inductive signaling that establishes discrete [email protected] bone centers. Changes in these relationships facilitate skull evolution and may create susceptibility to disease. These concepts provide a general framework for approaching issues of homology in Present address: †Department of Cell and Tissue Biology, cases where germ layer origins have shifted during evolution. University of California, San Francisco, San Francisco, United States Introduction Competing interests: The At the beginning of skull vault development, mesenchymal cells of either neural crest or mesoderm authors declare that no origin condense into nascent bone fields. -

Variability of the Parietal Foramen and the Evolution of the Pineal Eye in South African Permo-Triassic Eutheriodont Therapsids
The sixth sense in mammalian forerunners: Variability of the parietal foramen and the evolution of the pineal eye in South African Permo-Triassic eutheriodont therapsids JULIEN BENOIT, FERNANDO ABDALA, PAUL R. MANGER, and BRUCE S. RUBIDGE Benoit, J., Abdala, F., Manger, P.R., and Rubidge, B.S. 2016. The sixth sense in mammalian forerunners: Variability of the parietal foramen and the evolution of the pineal eye in South African Permo-Triassic eutheriodont therapsids. Acta Palaeontologica Polonica 61 (4): 777–789. In some extant ectotherms, the third eye (or pineal eye) is a photosensitive organ located in the parietal foramen on the midline of the skull roof. The pineal eye sends information regarding exposure to sunlight to the pineal complex, a region of the brain devoted to the regulation of body temperature, reproductive synchrony, and biological rhythms. The parietal foramen is absent in mammals but present in most of the closest extinct relatives of mammals, the Therapsida. A broad ranging survey of the occurrence and size of the parietal foramen in different South African therapsid taxa demonstrates that through time the parietal foramen tends, in a convergent manner, to become smaller and is absent more frequently in eutherocephalians (Akidnognathiidae, Whaitsiidae, and Baurioidea) and non-mammaliaform eucynodonts. Among the latter, the Probainognathia, the lineage leading to mammaliaforms, are the only one to achieve the complete loss of the parietal foramen. These results suggest a gradual and convergent loss of the photoreceptive function of the pineal organ and degeneration of the third eye. Given the role of the pineal organ to achieve fine-tuned thermoregulation in ecto- therms (i.e., “cold-blooded” vertebrates), the gradual loss of the parietal foramen through time in the Karoo stratigraphic succession may be correlated with the transition from a mesothermic metabolism to a high metabolic rate (endothermy) in mammalian ancestry. -

Semionotiform Fish from the Upper Jurassic of Tendaguru (Tanzania)
Mitt. Mus. Nat.kd. Berl., Geowiss. Reihe 2 (1999) 135-153 19.10.1999 Semionotiform Fish from the Upper Jurassic of Tendaguru (Tanzania) Gloria Arratial & Hans-Peter Schultze' With 12 figures Abstract The late Late Jurassic fishes collected by the Tendaguru expeditions (1909-1913) are represented only by a shark tooth and various specimens of the neopterygian Lepidotes . The Lepidotes is a new species characterized by a combination of features such as the presence of scattered tubercles in cranial bones of adults, smooth ganoid scales, two suborbital bones, one row of infraorbital bones, non-tritoral teeth, hyomandibula with an anteriorly expanded membranous outgrowth, two extrascapular bones, two postcleithra, and the absence of fringing fulcra on all fins. Key words: Fishes, Actinopterygii, Semionotiformes, Late Jurassic, East-Africa . Zusammenfassung Die spätoberjurassischen Fische, die die Tendaguru-Expedition zwischen 1909 und 1913 gesammelt hat, sind durch einen Haizahn und mehrere Exemplare des Neopterygiers Lepidotes repräsentiert. Eine neue Art der Gattung Lepidotes ist be- schrieben, sie ist durch eine Kombination von Merkmalen (vereinzelte Tuberkel auf den Schädelknochen adulter Tiere, glatte Ganoidschuppen, zwei Suborbitalia, eine Reihe von Infraorbitalia, nichttritoriale Zähne, Hyomandibulare mit einer membra- nösen nach vorne gerichteten Verbreiterung, zwei Extrascapularia, zwei Postcleithra und ohne sich gabelnde Fulkren auf dem Vorderrand der Flossen) gekennzeichnet. Schlüsselwörter: Fische, Actinopterygii, Semionotiformes, Oberer Jura, Ostafrika. Introduction margin, crescent shaped lateral line pore, and the number of scales in vertical and longitudinal At the excavations of the Tendaguru expeditions rows), and on the shape of teeth (non-tritoral) . (1909-1913), fish remains were collected to- However, the Tendaguru lepidotid differs nota- gether with the spectacular reptiles in sediments bly from L. -

A New Discosauriscid Seymouriamorph Tetrapod from the Lower Permian of Moravia, Czech Republic
A new discosauriscid seymouriamorph tetrapod from the Lower Permian of Moravia, Czech Republic JOZEF KLEMBARA Klembara, J. 2005. A new discosauriscid seymouriamorph tetrapod from the Lower Permian of Moravia, Czech Repub− lic. Acta Palaeontologica Polonica 50 (1): 25–48. A new genus and species, Makowskia laticephala gen. et sp. nov., of seymouriamorph tetrapod from the Lower Permian deposits of the Boskovice Furrow in Moravia (Czech Republic) is described in detail, and its cranial reconstruction is pre− sented. It is placed in the family Discosauriscidae (together with Discosauriscus and Ariekanerpeton) on the following character states: short preorbital region; rounded to oval orbits positioned mainly in anterior half of skull; otic notch dorsoventrally broad and anteroposteriorly deep; rounded to oval ventral scales. Makowskia is distinguished from other Discosauriscidae by the following characters: nasal bones equally long as broad; interorbital region broad; prefrontal− postfrontal contact lies in level of frontal mid−length (only from D. pulcherrimus); maxilla deepest at its mid−length; sub− orbital ramus of jugal short and dorsoventrally broad with long anterodorsal−posteroventral directed lacrimal−jugal su− ture; postorbital anteroposteriorly short and lacks elongated posterior process; ventral surface of basioccipital smooth; rows of small denticles placed on distinct ridges and intervening furrows radiate from place immediately laterally to artic− ular portion on ventral surface of palatal ramus of pterygoid (only from D. pulcherrimus); -
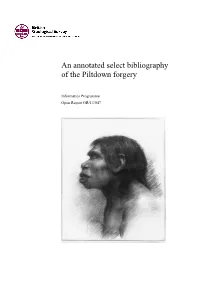
An Annotated Select Bibliography of the Piltdown Forgery
An annotated select bibliography of the Piltdown forgery Informatics Programme Open Report OR/13/047 BRITISH GEOLOGICAL SURVEY INFORMATICS PROGRAMME OPEN REPORT OR/13/47 An annotated select bibliography of the Piltdown forgery Compiled by David G. Bate Keywords Bibliography; Piltdown Man; Eoanthropus dawsoni; Sussex. Map Sheet 319, 1:50 000 scale, Lewes Front cover Hypothetical construction of the head of Piltdown Man, Illustrated London News, 28 December 1912. Bibliographical reference BATE, D. G. 2014. An annotated select bibliography of the Piltdown forgery. British Geological Survey Open Report, OR/13/47, iv,129 pp. Copyright in materials derived from the British Geological Survey’s work is owned by the Natural Environment Research Council (NERC) and/or the authority that commissioned the work. You may not copy or adapt this publication without first obtaining permission. Contact the BGS Intellectual Property Rights Section, British Geological Survey, Keyworth, e-mail [email protected]. You may quote extracts of a reasonable length without prior permission, provided a full acknowledgement is given of the source of the extract. © NERC 2014. All rights reserved Keyworth, Nottingham British Geological Survey 2014 BRITISH GEOLOGICAL SURVEY The full range of our publications is available from BGS shops at British Geological Survey offices Nottingham, Edinburgh, London and Cardiff (Welsh publications only) see contact details below or shop online at www. geologyshop.com BGS Central Enquiries Desk Tel 0115 936 3143 Fax 0115 936 3276 The London Information Office also maintains a reference collection of BGS publications, including maps, for consultation. email [email protected] We publish an annual catalogue of our maps and other publications; this catalogue is available online or from any of the Environmental Science Centre, Keyworth, Nottingham BGS shops.