The Effect of Lifelong Bilingualism on Regional Grey and White Matter Volume
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Reversal Sign Daniel Gaete,1 Antonio Lopez-Rueda2
Images in… BMJ Case Reports: first published as 10.1136/bcr-2014-204419 on 16 May 2014. Downloaded from The reversal sign Daniel Gaete,1 Antonio Lopez-Rueda2 1Clinica Alemana de Santiago, DESCRIPTION Santiago, Chile A 75-year-old man with a history of chronic 2Hospital Clinic i Provincial de Barcelona, Barcelona, Spain obstructive pulmonary disease was found in cardio- pulmonary arrest. After successful resuscitation the Correspondence to patient was transferred to our institution. On Antonio Lopez-Rueda, arrival, a non-enhanced brain CT was performed to [email protected] assess brain damage, which showed signs of diffuse Accepted 18 April 2014 cerebral oedema, with effacement of the cerebral sulci, sulcal hyperdensity and decreased attenuation of deep and cortical grey matter which appears hypodense in comparison to the white matter, a finding referred to as the ‘reversal sign’ (figures 1 and 2). These injuries were secondary to global brain ischaemia. In less than 8 h, the patient devel- oped multiple organ dysfunction syndrome and was pronounced dead. Cardiopulmonary arrest may lead to diffuse hypoxic ischaemic brain injury. Initially, unen- hanced brain CT may show subtle hypodensity of Figure 2 Coronal reformatted image of the same the basal ganglia and insular cortex, with efface- patient showing the reversal sign, which is also present ment of the basal cisterns. When diffuse brain in the cerebellum. oedema develops, the findings become more obvious, with effacement of the sulci and cisterns, The reversal sign reflects a diffuse hypoxic and loss of the grey matter–white matter differenti- ischaemic cerebral injury, with irreversible brain ation. -

Histological Underpinnings of Grey Matter Changes in Fibromyalgia Investigated Using Multimodal Brain Imaging
1090 • The Journal of Neuroscience, February 1, 2017 • 37(5):1090–1101 Neurobiology of Disease Histological Underpinnings of Grey Matter Changes in Fibromyalgia Investigated Using Multimodal Brain Imaging X Florence B. Pomares,1,2 Thomas Funck,3 Natasha A. Feier,1 XSteven Roy,1 X Alexandre Daigle-Martel,4 XMarta Ceko,5 Sridar Narayanan,3 XDavid Araujo,3 Alexander Thiel,6,7 Nikola Stikov,4,8 Mary-Ann Fitzcharles,9,10 and Petra Schweinhardt1,2,6,11 1Alan Edwards Centre for Research on Pain, McGill University, Montreal, Quebec H3A 0C7, Canada, 2Faculty of Dentistry, McGill University, Montreal, Quebec H3A 0C7, Canada, 3McConnell Brain Imaging Centre, Montreal Neurological Institute, McGill University, Montreal, Quebec, H3A 2B4, Canada, 4Institute for Biomedical Engineering, E´cole Polytechnique, Montreal, Quebec H3T 1J4, Canada, 5Institute of Cognitive Science, University of Colorado, Boulder, Colorado 80309, 6Department of Neurology & Neurosurgery, McGill University, Montreal, Quebec H3A 0C7, Canada, 7Jewish General Hospital, Montreal, Quebec H3T 1E2, Canada, 8Montreal Heart Institute, Montreal, Quebec, H1T 1C8, Canada, 9Division of Rheumatology, McGill University Health Centre, Montreal, Quebec H3G 1A4, Canada, 10Alan Edwards Pain Management Unit, McGill University Health Centre, Montreal, Quebec H3G 1A4, Canada, and 11Interdisciplinary Spinal Research, Department of Chiropractic Medicine, University Hospital Balgrist, 8008 Zurich, Switzerland Chronic pain patients present with cortical gray matter alterations, observed with anatomical magnetic resonance (MR) imaging. Reduced regional gray matter volumes are often interpreted to reflect neurodegeneration, but studies investigating the cellular origin of gray matter changesarelacking.Weusedmultimodalimagingtocompare26postmenopausalwomenwithfibromyalgiawith25healthycontrols(agerange: 50–75 years) to test whether regional gray matter volume decreases in chronic pain are associated with compromised neuronal integrity. -

RAL COLOR CHART ***** This Chart Is to Be Used As a Guide Only. Colors May Appear Slightly Different ***** Green Beige Purple V
RAL COLOR CHART ***** This Chart is to be used as a guide only. Colors May Appear Slightly Different ***** RAL 1000 Green Beige RAL 4007 Purple Violet RAL 7008 Khaki Grey RAL 4008 RAL 7009 RAL 1001 Beige Signal Violet Green Grey Tarpaulin RAL 1002 Sand Yellow RAL 4009 Pastel Violet RAL 7010 Grey RAL 1003 Signal Yellow RAL 5000 Violet Blue RAL 7011 Iron Grey RAL 1004 Golden Yellow RAL 5001 Green Blue RAL 7012 Basalt Grey Ultramarine RAL 1005 Honey Yellow RAL 5002 RAL 7013 Brown Grey Blue RAL 1006 Maize Yellow RAL 5003 Saphire Blue RAL 7015 Slate Grey Anthracite RAL 1007 Chrome Yellow RAL 5004 Black Blue RAL 7016 Grey RAL 1011 Brown Beige RAL 5005 Signal Blue RAL 7021 Black Grey RAL 1012 Lemon Yellow RAL 5007 Brillant Blue RAL 7022 Umbra Grey Concrete RAL 1013 Oyster White RAL 5008 Grey Blue RAL 7023 Grey Graphite RAL 1014 Ivory RAL 5009 Azure Blue RAL 7024 Grey Granite RAL 1015 Light Ivory RAL 5010 Gentian Blue RAL 7026 Grey RAL 1016 Sulfer Yellow RAL 5011 Steel Blue RAL 7030 Stone Grey RAL 1017 Saffron Yellow RAL 5012 Light Blue RAL 7031 Blue Grey RAL 1018 Zinc Yellow RAL 5013 Cobolt Blue RAL 7032 Pebble Grey Cement RAL 1019 Grey Beige RAL 5014 Pigieon Blue RAL 7033 Grey RAL 1020 Olive Yellow RAL 5015 Sky Blue RAL 7034 Yellow Grey RAL 1021 Rape Yellow RAL 5017 Traffic Blue RAL 7035 Light Grey Platinum RAL 1023 Traffic Yellow RAL 5018 Turquiose Blue RAL 7036 Grey RAL 1024 Ochre Yellow RAL 5019 Capri Blue RAL 7037 Dusty Grey RAL 1027 Curry RAL 5020 Ocean Blue RAL 7038 Agate Grey RAL 1028 Melon Yellow RAL 5021 Water Blue RAL 7039 Quartz Grey -

Green, Grey Or Green-Grey? Decoding Infrastructure Integration and Implementation for Residential Street Retrofits
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Green, Grey or Green-Grey? Decoding infrastructure integration and implementation for residential street retrofits. A thesis submitted in partial fulfilment of the requirements for the Degree of Master of Landscape Architecture at L I N C O L N U N I V E R S I T Y New Zealand by K S E N I A I. A L E K S A N D R O V A Lincoln University 2016 A B S T R A C T New-world countries are often characterized by large areas of sprawling post-war suburban development. The streets of these suburbs are often criticized for being unattractive transport corridors that prioritize cars at the cost of the pedestrian environment and ecological health of their neighbourhoods. Green infrastructure (LID) street retrofits, as well as grey infrastructure-dominated traffic calming schemes have been used as partial solutions to the adverse effects of post-war street design. There is, however, a lack of implementation of these measures, along with some confusion as to what is defined as green and grey infrastructure at the street scale. -

OBSERVATIONS on PARTIAL REMOVAL of the POST-CENTRAL GYRUS for PAIN by WALPOLE LEWIN and C
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.15.3.143 on 1 August 1952. Downloaded from J. Neurol. Neurosurg. Psychiat., 1952, 15, 143. OBSERVATIONS ON PARTIAL REMOVAL OF THE POST-CENTRAL GYRUS FOR PAIN BY WALPOLE LEWIN and C. G. PHILLIPS From the Nuffield Department ofSurgery and the University Laboratory ofPhysiology, Oxford The role of the cerebral cortex in the conscious one case where stimulation of the sensory cortex appreciation of pain has interested neurologists for produced pain in the phantom leg. many years. Head and Holmes (1911) considered In this paper we wish to record three cases in that pain entered consciousness at thalamic level, which partial resection of the post-central gyrus and more recently Penfleld (1947) stated that was undertaken for the relief of pain. In the first 6. .no removal of cortex anywhere can prevent patient the pain developed during an unusual, pain from being felt and only very rarely does a prolonged, sensory painful aura in traumatic patient use the word pain to describe the result of epilepsy, the second patient had intractable pain in cortical stimulation ", and, he goes on, " it is a phantom foot, and the third had a painful thigh obvious therefore that the pathway of pain conduc- stump. In all three, electrical stimulation of the Protected by copyright. tion reaches the thalamus and consciousness appropriate area of the post-central gyrus repro- without essential conduction to the cortex ". duced the pain complained of by the patient and Adrian (1941) found that no impulses reached the relief followed the removal of this area of cortex. -

Translingual Neural Stimulation with the Portable Neuromodulation
Translingual Neural Stimulation With the Portable Neuromodulation Stimulator (PoNS®) Induces Structural Changes Leading to Functional Recovery In Patients With Mild-To-Moderate Traumatic Brain Injury Authors: Jiancheng Hou,1 Arman Kulkarni,2 Neelima Tellapragada,1 Veena Nair,1 Yuri Danilov,3 Kurt Kaczmarek,3 Beth Meyerand,2 Mitchell Tyler,2,3 *Vivek Prabhakaran1 1. Department of Radiology, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wisconsin, USA 2. Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, Wisconsin, USA 3. Department of Kinesiology, University of Wisconsin-Madison, Madison, Wisconsin, USA *Correspondence to [email protected] Disclosure: Dr Tyler, Dr Danilov, and Dr Kaczmarek are co-founders of Advanced Neurorehabilitation, LLC, which holds the intellectual property rights to the PoNS® technology. Dr Tyler is a board member of NeuroHabilitation Corporation, a wholly- owned subsidiary of Helius Medical Technologies, and owns stock in the corporation. The other authors have declared no conflicts of interest. Acknowledgements: Professional medical writing and editorial assistance were provided by Kelly M. Fahrbach, Ashfield Healthcare Communications, part of UDG Healthcare plc, funded by Helius Medical Technologies. Dr Tyler, Dr Kaczmarek, Dr Danilov, Dr Hou, and Dr Prabhakaran were being supported by NHC-TBI-PoNS-RT001. Dr Hou, Dr Kulkarni, Dr Nair, Dr Tellapragada, and Dr Prabhakaran were being supported by R01AI138647. Dr Hou and Dr Prabhakaran were being supported by P01AI132132, R01NS105646. Dr Kulkarni was being supported by the Clinical & Translational Science Award programme of the National Center for Research Resources, NCATS grant 1UL1RR025011. Dr Meyerand, Dr Prabhakaran, Dr Nair was being supported by U01NS093650. -

Neocortex: Consciousness Cerebellum
Grey matter (chips) White matter (the wiring: the brain mainly talks to itself) Neocortex: consciousness Cerebellum: unconscious control of posture & movement brains 1. Golgi-stained section of cerebral cortex 2. One of Ramon y Cajal’s faithful drawings showing nerve cell diversity in the brain cajal Neuropil: perhaps 1 km2 of plasma membrane - a molecular reaction substrate for 1024 voltage- and ligand-gated ion channels. light to Glia: 3 further cell types 1. Astrocytes: trophic interface with blood, maintain blood brain barrier, buffer excitotoxic neurotransmitters, support synapses astros Oligodendrocytes: myelin insulation oligos Production persists into adulthood: radiation myelopathy 3. Microglia: resident macrophages of the CNS. Similarities and differences with Langerhans cells, the professional antigen-presenting cells of the skin. 3% of all cells, normally renewed very slowly by division and immigration. Normal Neurosyphilis microglia Most adult neurons are already produced by birth Peak synaptic density by 3 months EMBRYONIC POSTNATAL week: 0 6 12 18 24 30 36 Month: 0 6 12 18 24 30 36 Year: 4 8 12 16 20 24 Cell birth Migration 2* Neurite outgrowth Synaptogenesis Myelination 1* Synapse elimination Modified from various sources inc: Andersen SL Neurosci & Biobehav Rev 2003 Rakic P Nat Rev Neurosci 2002 Bourgeois Acta Pediatr Suppl 422 1997 timeline 1 Synaptogenesis 100% * Rat RTH D BI E A Density of synapses in T PUBERTY primary visual cortex H at different times post- 0% conception. 100% (logarithmic scale) RTH Cat BI D E A T PUBERTY H The density values equivalent 0% to 100% vary between species 100% but in Man the peak value is Macaque 6 3 RTH 350 x10 synapses per mm BI D E PUBERTY A T The peak rate of synapse H formation is at birth in the 0% macaque: extrapolating to 100% the entire cortex, this Man RTH BI amounts to around 800,000 D E synapses formed per sec. -

148 Colour Chart
148 colour chart IVORY PRIMROSE BUTTERCUP SOFT LIME TULIP YELLOW LEMON YELLOW CANARY SUNFLOWER ALMOND BLUSH SAFFRON Y418 Y919 Y417 Y828 Y337 Y747 Y657 Y367 Y156 O819 O729 O739 VANILLA PASTEL YELLOW MUSTARD OATMEAL APRICOT HONEYCOMB GOLD AMBER PUMPKIN GINGER BRIGHT ORANGE MANDARIN O929 O949 O948 O628 O538 O547 O555 O567 O467 O136 O177 O277 ORANGE SPICE BURNT ORANGE SATIN DUSKY PINK PUTTY SUNKISSED PINK CORAL SOFT PEACH PEACH MANGO PASTEL PINK R866 O346 R946 Y129 O518 O618 O228 R937 O138 O148 O248 R738 COCKTAIL PINK SALMON PINK ANTIQUE PINK LIPSTICK RED RED BERRY RED RUBY POPPY CRIMSON CARDINAL RED BURGUNDY MAROON R438 R547 R346 R576 R666 R665 R455 R565 R445 R244 R424 M544 PALE PINK BABY PINK ROSE PINK CERISE HOT PINK MAGENTA CARMINE DUSKY ROSE BLOSSOM PINK CARNATION FUCHSIA PINK SLATE R519 R228 M727 M647 R365 M865 R156 R327 M428 M328 M137 V715 AMETHYST PURPLE MULBERRY PLUM AUBERGINE LAVENDER ORCHID LILAC BLUEBELL VIOLET PRUSSIAN BLUE PEARL V626 V546 V865 V735 V524 V518 V528 V327 V127 V245 V464 B528 CORNFLOWER COBALT BLUE CHINA BLUE MIDNIGHT BLUE INDIGO BLUE ROYAL BLUE TRUE BLUE AZURE SKY BLUE CYAN PASTEL BLUE POWDER BLUE B617 B637 B736 B624 V234 V264 B555 B346 B137 C847 C719 B119 ARCTIC BLUE DENIM BLUE AEGEAN FRENCH NAVY COOL AQUA DUCK EGG TURQUOISE MARINE PETROL BLUE HOLLY PINE EMERALD B138 C917 B146 B445 C429 C528 C247 C446 C824 G724 G635 G657 GREEN LUSH GREEN PASTEL GREEN SOFT GREEN MINT GREEN GRASS FOREST GREEN TEA GREEN GREY GREEN MEADOW GREEN APPLE LEAF GREEN G847 G756 G829 G817 G637 G457 G356 G619 G917 G339 G338 G258 BRIGHT GREEN -

Color Chart Colorchart
Color Chart AMERICANA ACRYLICS Snow (Titanium) White White Wash Cool White Warm White Light Buttermilk Buttermilk Oyster Beige Antique White Desert Sand Bleached Sand Eggshell Pink Chiffon Baby Blush Cotton Candy Electric Pink Poodleskirt Pink Baby Pink Petal Pink Bubblegum Pink Carousel Pink Royal Fuchsia Wild Berry Peony Pink Boysenberry Pink Dragon Fruit Joyful Pink Razzle Berry Berry Cobbler French Mauve Vintage Pink Terra Coral Blush Pink Coral Scarlet Watermelon Slice Cadmium Red Red Alert Cinnamon Drop True Red Calico Red Cherry Red Tuscan Red Berry Red Santa Red Brilliant Red Primary Red Country Red Tomato Red Naphthol Red Oxblood Burgundy Wine Heritage Brick Alizarin Crimson Deep Burgundy Napa Red Rookwood Red Antique Maroon Mulberry Cranberry Wine Natural Buff Sugared Peach White Peach Warm Beige Coral Cloud Cactus Flower Melon Coral Blush Bright Salmon Peaches 'n Cream Coral Shell Tangerine Bright Orange Jack-O'-Lantern Orange Spiced Pumpkin Tangelo Orange Orange Flame Canyon Orange Warm Sunset Cadmium Orange Dried Clay Persimmon Burnt Orange Georgia Clay Banana Cream Sand Pineapple Sunny Day Lemon Yellow Summer Squash Bright Yellow Cadmium Yellow Yellow Light Golden Yellow Primary Yellow Saffron Yellow Moon Yellow Marigold Golden Straw Yellow Ochre Camel True Ochre Antique Gold Antique Gold Deep Citron Green Margarita Chartreuse Yellow Olive Green Yellow Green Matcha Green Wasabi Green Celery Shoot Antique Green Light Sage Light Lime Pistachio Mint Irish Moss Sweet Mint Sage Mint Mint Julep Green Jadeite Glass Green Tree Jade -

Unravelling the Subfields of the Hippocampal Head Using 7-Tesla Structural MRI
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 8-2-2016 12:00 AM Unravelling The Subfields Of The Hippocampal Head Using 7-Tesla Structural MRI Jordan M. K. DeKraker The University of Western Ontario Supervisor Dr. Stefan Kohler̈ The University of Western Ontario Graduate Program in Psychology A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © Jordan M. K. DeKraker 2016 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Cognitive Neuroscience Commons, Computational Neuroscience Commons, Nervous System Commons, Other Neuroscience and Neurobiology Commons, and the Tissues Commons Recommended Citation DeKraker, Jordan M. K., "Unravelling The Subfields Of The Hippocampal Head Using 7-Tesla Structural MRI" (2016). Electronic Thesis and Dissertation Repository. 3918. https://ir.lib.uwo.ca/etd/3918 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. ii Abstract Probing the functions of human hippocampal subfields is a promising area of research in cognitive neuroscience. However, defining subfield borders in Magnetic Resonance Imaging (MRI) is challenging. Here, we present a user-guided, semi-automated protocol for segmenting hippocampal subfields on T2-weighted images obtained with 7-Tesla MRI. The protocol takes advantage of extant knowledge about regularities in hippocampal morphology and ontogeny that have not been systematically considered in prior related work. An image feature known as the hippocampal ‘dark band’ facilitates tracking of subfield continuities, allowing for unfolding and segmentation of convoluted hippocampal tissue. -
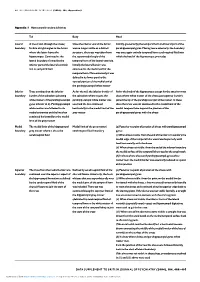
Appendix 1..1
BRITISH JOURNAL OF PSYCHIATRY (2004), 184, Appendix/1 Appendix 1 Hippocampal boundary definitions TailBody Head LateralLateral A line drawn through the alveus/ Once the inferior end of the fornix Initially (posteriorly) the temporal horn and lateral part of the boundary fimbria at right angles to the fornix was no longer visible as a distinct parahippocampal gyrus. Moving more anteriorly, the boundary where the latter leaves the structure, the trace was taken from was once again entirely temporal horn cerebrospinal fluid into hippocampus. Continued as the the superomedial angle of the which the head of the hippocampus protrudes lateral boundary, formed by the temporal horn of the lateral ventricle. inferior part of the lateral ventricle Initially the lateral border was and its temporal horn continued as the medial wall of the temporal horn. More anteriorly it was defined in its lower part by the vertical portion of the medial end of thetheparahippocampalwhitematter parahippocampal white matter Inferior Trace continued on the inferior As for the tail, the inferior border of As for the body of the hippocampus except for the anterior-most boundary border of the subiculum (abutting the subiculum where it joins the slices where white matter of the alveus (see superior border) white matter of the parahippocampal parahippocampal white matter was joined the tip of the parahippocampal white matter. In these gyrus inferior to it). Parahippocampal used and the line continued slices the trace was not continued to the medial limit of the white matter -

Flags and Symbols � � � Gilbert Baker Designed the Rainbow flag for the 1978 San Francisco’S Gay Freedom Celebration
Flags and Symbols ! ! ! Gilbert Baker designed the rainbow flag for the 1978 San Francisco’s Gay Freedom Celebration. In the original eight-color version, pink stood for sexuality, red for life, orange for healing, yellow for the sun, green for nature, turquoise for art, indigo for harmony and violet for the soul.! " Rainbow Flag First unveiled on 12/5/98 the bisexual pride flag was designed by Michael Page. This rectangular flag consists of a broad magenta stripe at the top (representing same-gender attraction,) a broad stripe in blue at the bottoms (representing opposite- gender attractions), and a narrower deep lavender " band occupying the central fifth (which represents Bisexual Flag attraction toward both genders). The pansexual pride flag holds the colors pink, yellow and blue. The pink band symbolizes women, the blue men, and the yellow those of a non-binary gender, such as a gender bigender or gender fluid Pansexual Flag In August, 2010, after a process of getting the word out beyond the Asexual Visibility and Education Network (AVEN) and to non-English speaking areas, a flag was chosen following a vote. The black stripe represents asexuality, the grey stripe the grey-are between sexual and asexual, the white " stripe sexuality, and the purple stripe community. Asexual Flag The Transgender Pride flag was designed by Monica Helms. It was first shown at a pride parade in Phoenix, Arizona, USA in 2000. The flag represents the transgender community and consists of five horizontal stripes. Two light blue which is the traditional color for baby boys, two pink " for girls, with a white stripe in the center for those Transgender Flag who are transitioning, who feel they have a neutral gender or no gender, and those who are intersex.