Simian Immunodeficiency Virus Envelope Glycoprotein Counteracts Tetherin/BST-2/CD317 by Intracellular Sequestration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of SAMHD1 in Restriction and Immune Sensing of Retroviruses and Retroelements
The role of SAMHD1 in restriction and immune sensing of retroviruses and retroelements Die Rolle von SAMHD1 in der Restriktion und Immunerkennung von Retroviren und Retroelementen Der Naturwissenschaftlichen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg zur Erlangung des Doktorgrades Dr. rer. nat. vorgelegt von Alexandra Herrmann aus Biberach an der Riß Als Dissertation genehmigt von der Naturwissenschaftlichen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg Tag der mündlichen Prüfung: 31.07.2018 Vorsitzender des Promotionsorgans: Prof. Dr. Georg Kreimer Gutachter: Prof. Dr. Lars Nitschke Prof. Dr. Manfred Marschall Table of content Table of content I. Summary ......................................................................................................................... 1 I. Zusammenfassung ......................................................................................................... 3 II. Introduction ..................................................................................................................... 5 1. The human immunodeficiency virus .................................................................................... 5 2. Transposable elements ......................................................................................................... 7 3. Host restriction factors ........................................................................................................ 10 4. The restriction factor SAMHD1 .......................................................................................... -

Canine Influenza Virus Is Mildly Restricted by Canine Tetherin Protein
viruses Article Canine Influenza Virus is Mildly Restricted by Canine Tetherin Protein Yun Zheng 1,2,3,†, Xiangqi Hao 1,2,3,†, Qingxu Zheng 1,2,3, Xi Lin 1,2,3, Xin Zhang 1,2,3, Weijie Zeng 1,2,3, Shiyue Ding 1, Pei Zhou 1,2,3,* and Shoujun Li 1,2,3,* 1 College of Veterinary Medicine, South China Agricultural University, Guangzhou 510642, China; [email protected] (Y.Z.); [email protected] (X.H.); [email protected] (Q.Z.); [email protected] (X.L.); [email protected] (X.Z.); [email protected] (W.Z.); [email protected] (S.D.) 2 Guangdong Provincial Key Laboratory of Prevention and Control for Severe Clinical Animal Diseases, Guangzhou 510642, China 3 Guangdong Provincial Pet Engineering Technology Research Center, Guangzhou 510642, China * Correspondence: [email protected] (P.Z.); [email protected] (S.L.) † These authors contributed equally to this work. Received: 10 July 2018; Accepted: 10 October 2018; Published: 16 October 2018 Abstract: Tetherin (BST2/CD317/HM1.24) has emerged as a key host-cell ·defence molecule that acts by inhibiting the release and spread of diverse enveloped virions from infected cells. We analysed the biological features of canine tetherin and found it to be an unstable hydrophilic type I transmembrane protein with one transmembrane domain, no signal peptide, and multiple glycosylation and phosphorylation sites. Furthermore, the tissue expression profile of canine tetherin revealed that it was particularly abundant in immune organs. The canine tetherin gene contains an interferon response element sequence that can be regulated and expressed by canine IFN-α. -

Tetherin/BST-2 Promotes Dendritic Cell Activation and Function During Acute Retrovirus Infection Received: 21 October 2015 Sam X
www.nature.com/scientificreports OPEN Tetherin/BST-2 promotes dendritic cell activation and function during acute retrovirus infection Received: 21 October 2015 Sam X. Li1,2, Bradley S. Barrett1, Kejun Guo1, George Kassiotis3, Kim J. Hasenkrug4, Accepted: 06 January 2016 Ulf Dittmer5, Kathrin Gibbert5 & Mario L. Santiago1,2 Published: 05 February 2016 Tetherin/BST-2 is a host restriction factor that inhibits retrovirus release from infected cells in vitro by tethering nascent virions to the plasma membrane. However, contradictory data exists on whether Tetherin inhibits acute retrovirus infection in vivo. Previously, we reported that Tetherin-mediated inhibition of Friend retrovirus (FV) replication at 2 weeks post-infection correlated with stronger natural killer, CD4+ T and CD8+ T cell responses. Here, we further investigated the role of Tetherin in counteracting retrovirus replication in vivo. FV infection levels were similar between wild-type (WT) and Tetherin KO mice at 3 to 7 days post-infection despite removal of a potent restriction factor, Apobec3/Rfv3. However, during this phase of acute infection, Tetherin enhanced myeloid dendritic cell (DC) function. DCs from infected, but not uninfected, WT mice expressed significantly higher MHC class II and the co-stimulatory molecule CD80 compared to Tetherin KO DCs. Tetherin-associated DC activation during acute FV infection correlated with stronger NK cell responses. Furthermore, Tetherin+ DCs from FV-infected mice more strongly stimulated FV-specific CD4+ T cells ex vivo compared to Tetherin KO DCs. The results link the antiretroviral and immunomodulatory activity of Tetherin in vivo to improved DC activation and MHC class II antigen presentation. Restriction factors are host-encoded type I interferon-stimulated genes (ISG) that directly inhibit virus replication1. -

Modulating Antiviral Response Via CRISPR–Cas Systems
viruses Review Immunity and Viral Infections: Modulating Antiviral Response via CRISPR–Cas Systems Sergey Brezgin 1,2,3,† , Anastasiya Kostyusheva 1,†, Ekaterina Bayurova 4 , Elena Volchkova 5, Vladimir Gegechkori 6 , Ilya Gordeychuk 4,7, Dieter Glebe 8 , Dmitry Kostyushev 1,3,*,‡ and Vladimir Chulanov 1,3,5,‡ 1 National Medical Research Center of Tuberculosis and Infectious Diseases, Ministry of Health, 127994 Moscow, Russia; [email protected] (S.B.); [email protected] (A.K.); [email protected] (V.C.) 2 Institute of Immunology, Federal Medical Biological Agency, 115522 Moscow, Russia 3 Scientific Center for Genetics and Life Sciences, Division of Biotechnology, Sirius University of Science and Technology, 354340 Sochi, Russia 4 Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products of Russian Academy of Sciences, 108819 Moscow, Russia; [email protected] (E.B.); [email protected] (I.G.) 5 Department of Infectious Diseases, Sechenov University, 119991 Moscow, Russia; [email protected] 6 Department of Pharmaceutical and Toxicological Chemistry, Sechenov University, 119991 Moscow, Russia; [email protected] 7 Department of Organization and Technology of Immunobiological Drugs, Sechenov University, 119991 Moscow, Russia 8 National Reference Center for Hepatitis B Viruses and Hepatitis D Viruses, Institute of Medical Virology, Justus Liebig University of Giessen, 35392 Giessen, Germany; [email protected] * Correspondence: [email protected] † Co-first authors. Citation: Brezgin, S.; Kostyusheva, ‡ Co-senior authors. A.; Bayurova, E.; Volchkova, E.; Gegechkori, V.; Gordeychuk, I.; Glebe, Abstract: Viral infections cause a variety of acute and chronic human diseases, sometimes resulting D.; Kostyushev, D.; Chulanov, V. Immunity and Viral Infections: in small local outbreaks, or in some cases spreading across the globe and leading to global pandemics. -

Hepatitis C Virus P7—A Viroporin Crucial for Virus Assembly and an Emerging Target for Antiviral Therapy
Viruses 2010, 2, 2078-2095; doi:10.3390/v2092078 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Hepatitis C Virus P7—A Viroporin Crucial for Virus Assembly and an Emerging Target for Antiviral Therapy Eike Steinmann and Thomas Pietschmann * TWINCORE †, Division of Experimental Virology, Centre for Experimental and Clinical Infection Research, Feodor-Lynen-Str. 7, 30625 Hannover, Germany; E-Mail: [email protected] † TWINCORE is a joint venture between the Medical School Hannover (MHH) and the Helmholtz Centre for Infection Research (HZI). * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +49-511-220027-130; Fax: +49-511-220027-139. Received: 22 July 2010; in revised form: 2 September 2010 / Accepted: 6 September 2010 / Published: 27 September 2010 Abstract: The hepatitis C virus (HCV), a hepatotropic plus-strand RNA virus of the family Flaviviridae, encodes a set of 10 viral proteins. These viral factors act in concert with host proteins to mediate virus entry, and to coordinate RNA replication and virus production. Recent evidence has highlighted the complexity of HCV assembly, which not only involves viral structural proteins but also relies on host factors important for lipoprotein synthesis, and a number of viral assembly co-factors. The latter include the integral membrane protein p7, which oligomerizes and forms cation-selective pores. Based on these properties, p7 was included into the family of viroporins comprising viral proteins from multiple virus families which share the ability to manipulate membrane permeability for ions and to facilitate virus production. Although the precise mechanism as to how p7 and its ion channel function contributes to virus production is still elusive, recent structural and functional studies have revealed a number of intriguing new facets that should guide future efforts to dissect the role and function of p7 in the viral replication cycle. -

Developing a Single-Cycle Infectious System to Study an ERV-K Retroviral Envelope
Developing a Single-Cycle Infectious System to Study an ERV-K Retroviral Envelope Author: Rana Elias Akleh Persistent link: http://hdl.handle.net/2345/bc-ir:107695 This work is posted on eScholarship@BC, Boston College University Libraries. Boston College Electronic Thesis or Dissertation, 2017 Copyright is held by the author. This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License (http:// creativecommons.org/licenses/by-nc-sa/4.0). Developing a Single-Cycle Infectious System to Study an ERV-K Retroviral Envelope Rana Elias Akleh A thesis submitted to the faculty of the department of Biology in partial fulfillment of the requirements for the degree of Master of Science Boston College Morrissey College of Arts and Sciences Graduate School September 2017 © 2017 Rana Elias Akleh Developing a Single-Cycle Infectious System to Study an ERV-K Retroviral Envelope Rana Elias Akleh Advisor: Welkin Johnson, Ph.D. Endogenous Retroviruses (ERVs) are “fossilized” retroviruses of a once exogenous retrovirus located in the genome of extant vertebrates. Retroviral infection results in a provirus integration into the host genome. An infection of a germline cell could lead to the provirus potentially being inherited by the offspring of the infected individual. Once in the genome, the provirus becomes subject to evolutionary processes and can become either lost or fixed in a population, remaining as “fossils” long after the exogenous retrovirus has gone extinct23. Notably, 8% of the human genome consists of ERVs30. Human Endogenous Retrovirus Type K (HERV-K)(HML-2) family is of particular interest. HERV-K integrations are as old as 30-35 million years, endogenizing before the separation of humans and Old World Monkeys. -

Endocytosis Elicited by Nectins Transfers Cytoplasmic Cargo, Including Infectious Material, Between Cells Alex R
© 2019. Published by The Company of Biologists Ltd | Journal of Cell Science (2019) 132, jcs235507. doi:10.1242/jcs.235507 RESEARCH ARTICLE Trans-endocytosis elicited by nectins transfers cytoplasmic cargo, including infectious material, between cells Alex R. Generous1,2, Oliver J. Harrison3, Regina B. Troyanovsky4, Mathieu Mateo1,*, Chanakha K. Navaratnarajah1, Ryan C. Donohue1,2, Christian K. Pfaller1,2, Olga Alekhina5, Alina P. Sergeeva3,6, Indrajyoti Indra4, Theresa Thornburg7,‡, Irina Kochetkova7, Daniel D. Billadeau5, Matthew P. Taylor7, Sergey M. Troyanovsky4, Barry Honig3,6, Lawrence Shapiro3 and Roberto Cattaneo1,2,§ ABSTRACT development, where the Bride of sevenless protein is internalized by the Sevenless tyrosine kinase receptor (Cagan et al., 1992). Here, we show that cells expressing the adherens junction protein Transfer of specific transmembrane proteins also occurs during nectin-1 capture nectin-4-containing membranes from the surface tissue patterning in embryonic development of higher of adjacent cells in a trans-endocytosis process. We find that vertebrates, during epithelial cell movement and at the immune internalized nectin-1–nectin-4 complexes follow the endocytic synapse (Gaitanos et al., 2016; Hudrisier et al., 2001; Marston et al., pathway. The nectin-1 cytoplasmic tail controls transfer: its deletion 2003; Matsuda et al., 2004; Qureshi et al., 2011). At the immune prevents trans-endocytosis, while its exchange with the nectin-4 tail synapse, the CTLA-4 protein captures its ligands CD80 and CD86 reverses transfer direction. Nectin-1-expressing cells acquire dye- from donor cells by a process of trans-endocytosis; after removal, labeled cytoplasmic proteins synchronously with nectin-4, a process these ligands are degraded inside the acceptor cell, resulting in most active during cell adhesion. -

Structural and Functional Analyses of the Interaction of Tetherin with the Dendritic Cell Receptor ILT7 Nicolas Aschman
Structural and functional analyses of the interaction of tetherin with the dendritic cell receptor ILT7 Nicolas Aschman To cite this version: Nicolas Aschman. Structural and functional analyses of the interaction of tetherin with the dendritic cell receptor ILT7. Biomolecules [q-bio.BM]. Université Grenoble Alpes, 2015. English. NNT : 2015GREAV064. tel-01682995 HAL Id: tel-01682995 https://tel.archives-ouvertes.fr/tel-01682995 Submitted on 12 Jan 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. #$ !"#$% '!"(%")!*+%"+% %&'$()*%$*+,(-./$)#.0*%$*2)$-&3+$ ,-.0'*('$."1"34565748*#9:;<9;:=68*89*-=>5?4565748 2!!3$."5'&'6$.!'%("1"7"*8$"9::; !.6% $.%"-*! -4<56=@*A#'BA- <=>6%"+'!').%"-*!"(%"C:"D4>E:48F*D$.##$-&)- -!.-*!.%"* "6%' "+%"( (>49*5E*/4:; *5 9*'866*.>98:=<945> +* 6"6G0<568*%5<95:=68*'(4I48*89*#<48><8 *F;*/4J=>9 A>=6K 8 * 9:;<9;:8668 *89* E5><945>>8668 *F8*6,4>9,:=<945>* F8*6=*9,9(8:4>8*=J8<*68*:,<8N98;: .+. <=>6%"6 $% %"- #('A %5% $"(%*P0*=J:46*P12TB +%C* $"(%"D !E"05-6."+%"1" %:*A>F:8=*'.BA)$++. FG*--!$% !( %:*4J8 *2A(%.- FG*--!$% !( C:*B=:<*5AB.- -

Researchers Unveil New Monkey Model for HIV 2 March 2009
Researchers unveil new monkey model for HIV 2 March 2009 By altering just one gene in HIV-1, scientists have evolving genes, APOBEC3 and TRIM5, which succeeded in infecting pig-tailed macaque produce unusual classes of defensive proteins with monkeys with a human version of the virus that distinctive capabilities to fight retroviruses such as has until now been impossible to study directly in HIV. These genes, shared by humans and their animals. The new strain of HIV has already been simian forebears, have evolved mutations specific used to demonstrate one method for preventing to each species' unique history of retroviral battles. infection and, with a little tweaking, could be a In most simians, the APOBEC3 and TRIM5 valuable model for vetting vaccine candidates. proteins actually kill HIV on sight, making it impossible for researchers to study the virus in an A team of researchers led by Paul Bieniasz and animal model. Instead, they have studied HIV's Theodora Hatziioannou at The Rockefeller cousin, simian immunodeficiency virus (SIV), which University showed that two pig-tailed macaques, causes an AIDS-like disease in certain monkey given a common antiretroviral treatment one week species. But SIV shares only about half of its amino before and one week after being exposed to the acid sequence with HIV, making it a very imperfect newly engineered HIV, had no signs of infection. substitute for testing anti-HIV drugs and vaccines. "We're not saying we can save the world with Several labs have engineered hybrids called SHIVs antiretroviral pills. But this model will allow us to — SIVs that contain pieces of HIV DNA — but these start studying the best way to administer have problematic differences, too. -

Tetherin-Mediated Restriction of Filovirus Budding Is Antagonized by the Ebola Glycoprotein
Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein Rachel L. Kaletsky, Joseph R. Francica, Caroline Agrawal-Gamse, and Paul Bates1 Department of Microbiology, School of Medicine, University of Pennsylvania, 225 Johnson Pavilion, 3610 Hamilton Walk, Philadelphia, PA 19104-6076 Edited by Stephen P. Goff, Columbia University College of Physicians and Surgeons, New York, NY, and approved December 19, 2008 (received for review October 31, 2008) Mammalian cells employ numerous innate cellular mechanisms to Ebola Zaire is a highly pathogenic negative-sense RNA virus inhibit viral replication and spread. Tetherin, also known as Bst-2 that causes severe hemorrhagic disease. Ebola belongs to the or CD317, is a recently identified, IFN-induced, cellular response family Filoviridae, whose members, the Ebola and Marburg factor that blocks release of HIV-1 and other retroviruses from viruses, produce filamentous virions. Expression of the Ebola infected cells. The means by which tetherin retains retroviruses on matrix protein, VP40, alone in mammalian cells produces fila- the cell surface, as well as the mechanism used by the HIV-1 mentous virus-like particles (VLPs) that bud from the cell accessory protein Vpu to antagonize tetherin function and pro- surface and structurally resemble infectious Ebola virions (6, 7). mote HIV-1 release, are unknown. Here, we document that tetherin The viral glycoprotein (GP) forms the surface spikes seen in the functions as a broadly acting antiviral factor by demonstrating that viral envelope membrane. Coexpression of Ebola GP with VP40 both human and murine tetherin potently inhibit the release of the produces VLPs that incorporate GP, resemble authentic Ebola filovirus, Ebola, from the surface of cells. -

APICAL M2 PROTEIN IS REQUIRED for EFFICIENT INFLUENZA a VIRUS REPLICATION by Nicholas Wohlgemuth a Dissertation Submitted To
APICAL M2 PROTEIN IS REQUIRED FOR EFFICIENT INFLUENZA A VIRUS REPLICATION by Nicholas Wohlgemuth A dissertation submitted to Johns Hopkins University in conformity with the requirements for the degree of Doctor of Philosophy Baltimore, Maryland October, 2017 © Nicholas Wohlgemuth 2017 All rights reserved ABSTRACT Influenza virus infections are a major public health burden around the world. This dissertation examines the influenza A virus M2 protein and how it can contribute to a better understanding of influenza virus biology and improve vaccination strategies. M2 is a member of the viroporin class of virus proteins characterized by their predicted ion channel activity. While traditionally studied only for their ion channel activities, viroporins frequently contain long cytoplasmic tails that play important roles in virus replication and disruption of cellular function. The currently licensed live, attenuated influenza vaccine (LAIV) contains a mutation in the M segment coding sequence of the backbone virus which confers a missense mutation (alanine to serine) in the M2 gene at amino acid position 86. Previously discounted for not showing a phenotype in immortalized cell lines, this mutation contributes to both the attenuation and temperature sensitivity phenotypes of LAIV in primary human nasal epithelial cells. Furthermore, viruses encoding serine at M2 position 86 induced greater IFN-λ responses at early times post infection. Reversing mutations such as this, and otherwise altering LAIV’s ability to replicate in vivo, could result in an improved LAIV development strategy. Influenza viruses infect at and egress from the apical plasma membrane of airway epithelial cells. Accordingly, the virus transmembrane proteins, HA, NA, and M2, are all targeted to the apical plasma membrane ii and contribute to egress. -
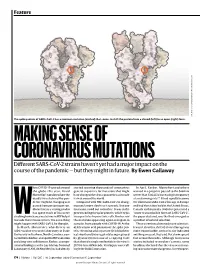
MAKING SENSE of CORONAVIRUS MUTATIONS Different SARS-Cov-2 Strains Haven’T Yet Had a Major Impact on the Course of the Pandemic — but They Might in Future
Feature SOURCE: STRUCTURAL DATA FROM K. SHEN & J. LUBAN K. SHEN FROM DATA STRUCTURAL SOURCE: The spike protein of SARS-CoV-2 has a common mutation (circled) that seems to shift the protein from a closed (left) to an open (right) form. MAKING SENSE OF CORONAVIRUS MUTATIONS Different SARS-CoV-2 strains haven’t yet had a major impact on the course of the pandemic — but they might in future. By Ewen Callaway hen COVID-19 spread around started scouring thousands of coronavirus In April, Korber, Montefiori and others the globe this year, David genetic sequences for mutations that might warned in a preprint posted to the bioRxiv Montefiori wondered how the have changed the virus’s properties as it made server that “D614G is increasing in frequency deadly virus behind the pan- its way around the world. at an alarming rate”1. It had rapidly become demic might be changing as it Compared with HIV, SARS-CoV-2 is chang- the dominant SARS-CoV-2 lineage in Europe passed from person to person. ing much more slowly as it spreads. But one and had then taken hold in the United States, Montefiori is a virologist who mutation stood out to Korber. It was in the Canada and Australia. D614G represented a has spent much of his career gene encoding the spike protein, which helps “more transmissible form of SARS-CoV-2”, Wstudying how chance mutations in HIV help it virus particles to penetrate cells. Korber saw the paper declared, one that had emerged as to evade the immune system.