Synthesis, Characterisation and Applications of Iron Oxide Nanoparticles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
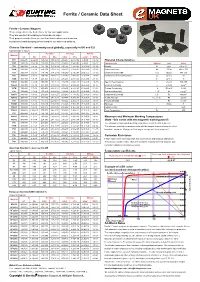
Ferrite / Ceramic Data Sheet
Ferrite / Ceramic Data Sheet Ferrite / Ceramic Magnets These magnets are the best choice for low cost applications. They are excellent at resisting corrosion due to water. Their properties make them an excellent choice when used in motors, loudspeakers and clamping devices and for use with reed switches. Chinese Standard - commonly used globally, especially in UK and EU Typical Range of Values Br Hc (Hcb) Hci (Hcj) BHmax Material mT kG kA/m kOe kA/m kOe kJ/m3 MGOe Y8T 200-235 2.0-2.35 125-160 1.57-2.01 210-280 2.64-3.52 6.5-9.5 0.8-1.2 Physical Characteristics Y10T 200-235 2.0-2.35 128-160 1.61-2.01 210-280 2.64-3.52 6.4-9.6 0.8-1.2 Characteristic Symbol Unit Value Y20 320-380 3.2-3.8 135-190 1.70-2.39 140-195 1.76-2.45 18.0-22.0 2.3-2.8 Density D g/cc 4.9 to 5.1 Y22H 310-360 3.1-3.6 220-250 2.76-3.14 280-320 3.52-4.02 20.0-24.0 2.5-3.0 Vickers Hardness Hv D.P.N 400 to 700 Y23 320-370 3.2-3.7 170-190 2.14-2.39 190-230 2.39-2.89 20.0-25.5 2.5-3.2 Compression Strength C.S N/mm2 680-720 Y25 360-400 3.6-4.0 135-170 1.70-2.14 140-200 1.76-2.51 22.5-28.0 2.8-3.5 Coefficient of Thermal Expansion C// 10-6/C 15 Y26H 360-390 3.6-3.9 220-250 2.76-3.14 225-255 2.83-3.20 23.0-28.0 2.9-3.5 C^ 10-6/C 10 Y26H-1 360-390 3.6-3.9 200-250 2.51-3.14 225-255 2.83-3.20 23.0-28.0 2.9-3.5 Specific Heat Capacity c J/kg°C 795-855 Y26H-2 360-380 3.6-3.8 263-288 3.30-3.62 318-350 4.00-4.40 24.0-28.0 3.0-3.5 Electrical Resistivity r m Ω.cm 1x1010 Y27H 370-400 3.7-4.0 205-250 2.58-3.14 210-255 2.64-3.20 25.0-29.0 3.1-3.6 Thermal Conductivity k W/cm°C 0.029 Y28 370-400 3.7-4.0 175-210 2.20-2.64 180-220 2.26-2.76 26.0-30.0 3.3-3.8 Modulus of Elasticity l / E Pa 1.8x1011 Y28H-1 380-400 3.8-4.0 240-260 3.02-3.27 250-280 3.14-3.52 27.0-30.0 3.4-3.8 Compression Strength C.S. -

Canted Ferrimagnetism and Giant Coercivity in the Non-Stoichiometric
Canted ferrimagnetism and giant coercivity in the non-stoichiometric double perovskite La2Ni1.19Os0.81O6 Hai L. Feng1, Manfred Reehuis2, Peter Adler1, Zhiwei Hu1, Michael Nicklas1, Andreas Hoser2, Shih-Chang Weng3, Claudia Felser1, Martin Jansen1 1Max Planck Institute for Chemical Physics of Solids, Dresden, D-01187, Germany 2Helmholtz-Zentrum Berlin für Materialien und Energie, Berlin, D-14109, Germany 3National Synchrotron Radiation Research Center (NSRRC), Hsinchu, 30076, Taiwan Abstract: The non-stoichiometric double perovskite oxide La2Ni1.19Os0.81O6 was synthesized by solid state reaction and its crystal and magnetic structures were investigated by powder x-ray and neutron diffraction. La2Ni1.19Os0.81O6 crystallizes in the monoclinic double perovskite structure (general formula A2BB’O6) with space group P21/n, where the B site is fully occupied by Ni and the B’ site by 19 % Ni and 81 % Os atoms. Using x-ray absorption spectroscopy an Os4.5+ oxidation state was established, suggesting presence of about 50 % 5+ 3 4+ 4 paramagnetic Os (5d , S = 3/2) and 50 % non-magnetic Os (5d , Jeff = 0) ions at the B’ sites. Magnetization and neutron diffraction measurements on La2Ni1.19Os0.81O6 provide evidence for a ferrimagnetic transition at 125 K. The analysis of the neutron data suggests a canted ferrimagnetic spin structure with collinear Ni2+ spin chains extending along the c axis but a non-collinear spin alignment within the ab plane. The magnetization curve of La2Ni1.19Os0.81O6 features a hysteresis with a very high coercive field, HC = 41 kOe, at T = 5 K, which is explained in terms of large magnetocrystalline anisotropy due to the presence of Os ions together with atomic disorder. -

Magnetism, Magnetic Properties, Magnetochemistry
Magnetism, Magnetic Properties, Magnetochemistry 1 Magnetism All matter is electronic Positive/negative charges - bound by Coulombic forces Result of electric field E between charges, electric dipole Electric and magnetic fields = the electromagnetic interaction (Oersted, Maxwell) Electric field = electric +/ charges, electric dipole Magnetic field ??No source?? No magnetic charges, N-S No magnetic monopole Magnetic field = motion of electric charges (electric current, atomic motions) Magnetic dipole – magnetic moment = i A [A m2] 2 Electromagnetic Fields 3 Magnetism Magnetic field = motion of electric charges • Macro - electric current • Micro - spin + orbital momentum Ampère 1822 Poisson model Magnetic dipole – magnetic (dipole) moment [A m2] i A 4 Ampere model Magnetism Microscopic explanation of source of magnetism = Fundamental quantum magnets Unpaired electrons = spins (Bohr 1913) Atomic building blocks (protons, neutrons and electrons = fermions) possess an intrinsic magnetic moment Relativistic quantum theory (P. Dirac 1928) SPIN (quantum property ~ rotation of charged particles) Spin (½ for all fermions) gives rise to a magnetic moment 5 Atomic Motions of Electric Charges The origins for the magnetic moment of a free atom Motions of Electric Charges: 1) The spins of the electrons S. Unpaired spins give a paramagnetic contribution. Paired spins give a diamagnetic contribution. 2) The orbital angular momentum L of the electrons about the nucleus, degenerate orbitals, paramagnetic contribution. The change in the orbital moment -

A TWO DIMENSIONAL FERRITE-CORE MEMORY Bv M
A TWO DIMENSIONAL FERRITE-CORE MEMORY Bv M. M. FAROOQUI, S. P. SKiVASTAVA AND R. N. NEOGI (Tata Institute of Fundamental Research, Bomba),) Received January t0, 1957 (Communicated by Prof. Bernard P ABS'IXACT This paper describes a two dimensional matrix memory using ferrite- cores. A urtit consisting of 100 words of I 1 binary digits each has been cortstructed for parallel operation. The word drive is from a biasr switch-core matrix, while the digit drive makes use of pulse--trans- formers. Logic and circuit techniques enable high discrimination bet- ween wanted and unwanted signals. A semi-automatic method for testing the memory cores is also described. INTRODUCTION THE potentiality of magnetie fer¡ with a rectangular hysteresis loop as binary storage elements for digital computer was realised earlier by Forrester. x Later, Papian% s and almost simultaneously Rajchman4, ~ showed the practicability of such a system by successfully operating memories of fairly large capacity. This paper describes a modest attempt in this direction. A memory of I00 words, 11 bits each, has beca constructed to operate with a smaU digital computer at the Tata institute of Fundamental Research, Bombay. THE PRINClPLE OF OPERATION OF THE MEMORY The two stable states required for sto¡ binary information in magnetic cores ate the states of positive and negative magnetisation. These are the states corresponding to the position A0 and Ax on the hysteresis loop and can be termed as the 0- and the 1-states respectively (Fig. 2). Con- sider a number of sucia cores with ah ideaUy rectangular hysteresis character- istic, arranged in rows and columns in the form of a matrix. -

Chapter 6 Antiferromagnetism and Other Magnetic Ordeer
Chapter 6 Antiferromagnetism and Other Magnetic Ordeer 6.1 Mean Field Theory of Antiferromagnetism 6.2 Ferrimagnets 6.3 Frustration 6.4 Amorphous Magnets 6.5 Spin Glasses 6.6 Magnetic Model Compounds TCD February 2007 1 1 Molecular Field Theory of Antiferromagnetism 2 equal and oppositely-directed magnetic sublattices 2 Weiss coefficients to represent inter- and intra-sublattice interactions. HAi = n’WMA + nWMB +H HBi = nWMA + n’WMB +H Magnetization of each sublattice is represented by a Brillouin function, and each falls to zero at the critical temperature TN (Néel temperature) Sublattice magnetisation Sublattice magnetisation for antiferromagnet TCD February 2007 2 Above TN The condition for the appearance of spontaneous sublattice magnetization is that these equations have a nonzero solution in zero applied field Curie Weiss ! C = 2C’, P = C’(n’W + nW) TCD February 2007 3 The antiferromagnetic axis along which the sublattice magnetizations lie is determined by magnetocrystalline anisotropy Response below TN depends on the direction of H relative to this axis. No shape anisotropy (no demagnetizing field) TCD February 2007 4 Spin Flop Occurs at Hsf when energies of paralell and perpendicular configurations are equal: HK is the effective anisotropy field i 1/2 This reduces to Hsf = 2(HKH ) for T << TN Spin Waves General: " n h q ~ q ! M and specific heat ~ Tq/n Antiferromagnet: " h q ~ q ! M and specific heat ~ Tq TCD February 2007 5 2 Ferrimagnetism Antiferromagnet with 2 unequal sublattices ! YIG (Y3Fe5O12) Iron occupies 2 crystallographic sites one octahedral (16a) & one tetrahedral (24d) with O ! Magnetite(Fe3O4) Iron again occupies 2 crystallographic sites one tetrahedral (8a – A site) & one octahedral (16d – B site) 3 Weiss Coefficients to account for inter- and intra-sublattice interaction TCD February 2007 6 Below TN, magnetisation of each sublattice is zero. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Magnetics in Switched-Mode Power Supplies Agenda
Magnetics in Switched-Mode Power Supplies Agenda • Block Diagram of a Typical AC-DC Power Supply • Key Magnetic Elements in a Power Supply • Review of Magnetic Concepts • Magnetic Materials • Inductors and Transformers 2 Block Diagram of an AC-DC Power Supply Input AC Rectifier PFC Input Filter Power Trans- Output DC Outputs Stage former Circuits (to loads) 3 Functional Block Diagram Input Filter Rectifier PFC L + Bus G PFC Control + Bus N Return Power StageXfmr Output Circuits + 12 V, 3 A - + Bus + 5 V, 10 A - PWM Control + 3.3 V, 5 A + Bus Return - Mag Amp Reset 4 Transformer Xfmr CR2 L3a + C5 12 V, 3 A CR3 - CR4 L3b + Bus + C6 5 V, 10 A CR5 Q2 - + Bus Return • In forward converters, as in most topologies, the transformer simply transmits energy from primary to secondary, with no intent of energy storage. • Core area must support the flux, and window area must accommodate the current. => Area product. 4 3 ⎛ PO ⎞ 4 AP = Aw Ae = ⎜ ⎟ cm ⎝ K ⋅ΔB ⋅ f ⎠ 5 Output Circuits • Popular configuration for these CR2 L3a voltages---two secondaries, with + From 12 V 12 V, 3 A a lower voltage output derived secondary CR3 C5 - from the 5 V output using a mag CR4 L3b + amp postregulator. From 5 V 5 V, 10 A secondary CR5 C6 - CR6 L4 SR1 + 3.3 V, 5 A CR8 CR7 C7 - Mag Amp Reset • Feedback to primary PWM is usually from the 5 V output, leaving the +12 V output quasi-regulated. 6 Transformer (cont’d) • Note the polarity dots. Xfmr CR2 L3a – Outputs conduct while Q2 is on. -

Ferrite and Metal Composite Inductors
Ferrite and Metal Composite Inductors Design and Characteristics © 2019 KEMET Corporation What is an Inductor? Coil Magnetic Magnetic flux φ (Wire) Field dφ Core e = - dt Material i The coil converts electric energy into magnetic energy and stores it. e Core Material Current through the coil of wire creates a magnetic field Air Ferrite Metal and stores it. (None) (Iron) Composite Different core materials change magnetic field strength. © 2019 KEMET Corporation Ferrite Inductor or Metal Composite Inductor? Ferrite InductorFerrite Metal InductorMetal Composite MaterialMaterial Type type Ni-ZnNi-Zn Mn-Zn Mn-Zn Fe based Fe Based Very Good!! No Good.. InductanceInductace GoodGood! Very Good No Good Very Good!! Magnetic Saturation Good! No Good.. Good! No Good.. Very Good!! MagneticThermal Saturation Property Good No Good Very Good Good! Very Good!! Good! Efficiency Very Good!! No Good.. Good! ThermalResistance Property of core Good No Good Very Good Efficiency SBC/SBCPGood TPI Very GoodMPC, MPCV Good Products MPLC, MPLCV Resistance of Core Very Good No Good Good © 2019 KEMET Corporation Ferrite and Metal Composite Comparison Advantage of Ferrite 1. Higher inductance with high permeability 2. Stable inductance in the right range High L and Low DCR capability Advantage of Metal Composite 1. Very slow saturation 2. Very stable saturation for the thermal Core Loss Comparison Good for Auto app especially Advantage of Ferrite Very low core loss in dynamic frequency range Mn-Zn Ferrite Metal Core Low power consumption capability © 2019 KEMET Corporation -

Switch Mode Power Supplies and Their Magnetics Tutorial
Datatronic Switch Mode Power Supplies and their Magnetics Many factors must be considered by designers when choosing the magnetic components required in today’s electronic power supplies DATATRONIC DISTRIBUTION, INC. Datatronic In today’s day and age the most often used topology for electronic power supplies is that of the Switch Mode Power Supply (SMPS), which is a major user of magnetics. In some applications the “older type” linear supplies are still used, but in the early 70’s SMPS came into being spurred by the development of faster switching transistors. This facilitated the use of much smaller magnetic components and greater efficiencies. DATATRONIC DISTRIBUTION, INC. Datatronic SMPS and their General Magnetic Usage In general, there are four different types of magnetic components that are needed for the typical SMPS. They include the Output Transformer, usually the most noticeable because of its size compared to the others, the Output Inductors, the Input Inductors and the Current Sense Transformer, each with its own important function. DATATRONIC DISTRIBUTION, INC. Datatronic SMPS and their General Magnetic Usage 1.The Output Transformer or “Main” Transformer takes the input voltage that is supplied to its primary winding and then transforms the input voltage to one or more voltages that are the output of the secondary winding or windings. 2. The Output Inductors are used to filter the output voltage so that the load “sees” a filtered DC voltage. DATATRONIC DISTRIBUTION, INC. Datatronic SMPS and their General Magnetic Usage 3. The Input Inductors filter out the noise generated by the switching transistors so that this noise isn’t emitted back to the source. -

Magnetism in Transition Metal Complexes
Magnetism for Chemists I. Introduction to Magnetism II. Survey of Magnetic Behavior III. Van Vleck’s Equation III. Applications A. Complexed ions and SOC B. Inter-Atomic Magnetic “Exchange” Interactions © 2012, K.S. Suslick Magnetism Intro 1. Magnetic properties depend on # of unpaired e- and how they interact with one another. 2. Magnetic susceptibility measures ease of alignment of electron spins in an external magnetic field . 3. Magnetic response of e- to an external magnetic field ~ 1000 times that of even the most magnetic nuclei. 4. Best definition of a magnet: a solid in which more electrons point in one direction than in any other direction © 2012, K.S. Suslick 1 Uses of Magnetic Susceptibility 1. Determine # of unpaired e- 2. Magnitude of Spin-Orbit Coupling. 3. Thermal populations of low lying excited states (e.g., spin-crossover complexes). 4. Intra- and Inter- Molecular magnetic exchange interactions. © 2012, K.S. Suslick Response to a Magnetic Field • For a given Hexternal, the magnetic field in the material is B B = Magnetic Induction (tesla) inside the material current I • Magnetic susceptibility, (dimensionless) B > 0 measures the vacuum = 0 material response < 0 relative to a vacuum. H © 2012, K.S. Suslick 2 Magnetic field definitions B – magnetic induction Two quantities H – magnetic intensity describing a magnetic field (Système Internationale, SI) In vacuum: B = µ0H -7 -2 µ0 = 4π · 10 N A - the permeability of free space (the permeability constant) B = H (cgs: centimeter, gram, second) © 2012, K.S. Suslick Magnetism: Definitions The magnetic field inside a substance differs from the free- space value of the applied field: → → → H = H0 + ∆H inside sample applied field shielding/deshielding due to induced internal field Usually, this equation is rewritten as (physicists use B for H): → → → B = H0 + 4 π M magnetic induction magnetization (mag. -

INTRODUCTION:- Ferrites Are Ferromagnetic Material Containing Predominantly Oxides Iron Along with Other Oxides of Barium, Stron
INTRODUCTION:- Ferrites are ferromagnetic material containing predominantly oxides iron along with other oxides of barium, strontium, manganese, nickel, zinc, lithium and cadmium .Ferrites are ideally suited for making device like inductor core, circulators, memory devices and also for various microwave application. Although the saturation magnetisation of ferrites less than that of ferromagnetic alloys, they have advantages such as applicability at higher frequency, lower price and greater electrical resistance. Since 1950, soft ferrites have been widely studied and have become a field of interest of many researches because of their application potential in the modern electronics industry. The electrical and magnetic properties of these materials are structure sensitive and can be altered by doping or substitution. The substitution of aluminium in the inverse ferrites like Nickel, copper and cobalt ferrites have proved to be useful by increasing their saturation, magnetization, resistivity, however, at the cost of decrease of Curie temperature. Cadmium ferrite is a normal spinel ; its magnetic moment per unit cell is zero. Low magnetic high resistive ferrite are so applicable in the high frequency transformer cores. The addition of aluminium which has strong preference for the octahedral sites should exhibit the decrease of the magnetisation because aluminium is nonmagnetic. As aluminium content increases, the magnetic moment of unit cell decreases that means show triangular spin moment, their occurs a reduction in the sub lattice interaction. The removal of iron ions from magnetic sublattice and substitution of the nonmagnetic aluminium ion in its place weakens the magnetism. Due to this substitution improves catalytic, dielectric and magnetic properties, as they possess high resistivity and negligible eddy current losses. -

Ferrite EMI Cable Cores Electro-Magnetic Interference Solutions
global solutions : local support ™ Ferrite EMI Cable Cores Electro-Magnetic Interference Solutions www.lairdtech.com Laird Technologies is the world leader in the design and supply of customized performance critical products for wireless and other advanced electronic applications. Laird Technologies partners with it's customers to help find solutions for applications in various industries such as: Aerospace Automotive Electronics Computers Consumer Electronics Data Communications Medical Equipment Military Network Equipment Telecommunications Laird Technologies offers its customers unique product solutions, dedication to research and development and a seamless network of manufacturing and customer support facilities located all across the globe. global solutions : local support ™ www.lairdtech.com Contents Ferrite Material Impedance Comparison ................................. 4 Design & Selection “Rules of Thumb” ..................................... 4 High Frequency (HFB-) Cylindrical Cores .................................. 5 High Frequency (HFA-) Split, Snap-On Cores ........................... 6 Broadband (28B-) Cylindrical Cores ........................................ 7 Broadband (28A-) Split, Snap-On Cores ................................ 10 Sorted Quick Reference Charts Broadband (28B- and 28A-) Cable Cores .......................... 12 Low Frequency (LFB-) Cylinderical Cores ............................... 14 Broadband (28R-) Ribbon & Flex Cable Cores ....................... 15 Broadband (28S-) Split Ribbion & Flex Cable Cores