Evidence Review E: Postnatal Management of Hypertension
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn Et Al
US 2004O224012A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn et al. (43) Pub. Date: Nov. 11, 2004 (54) TOPICAL APPLICATION AND METHODS Related U.S. Application Data FOR ADMINISTRATION OF ACTIVE AGENTS USING LIPOSOME MACRO-BEADS (63) Continuation-in-part of application No. 10/264,205, filed on Oct. 3, 2002. (76) Inventors: Pichit Suvanprakorn, Bangkok (TH); (60) Provisional application No. 60/327,643, filed on Oct. Tanusin Ploysangam, Bangkok (TH); 5, 2001. Lerson Tanasugarn, Bangkok (TH); Suwalee Chandrkrachang, Bangkok Publication Classification (TH); Nardo Zaias, Miami Beach, FL (US) (51) Int. CI.7. A61K 9/127; A61K 9/14 (52) U.S. Cl. ............................................ 424/450; 424/489 Correspondence Address: (57) ABSTRACT Eric G. Masamori 6520 Ridgewood Drive A topical application and methods for administration of Castro Valley, CA 94.552 (US) active agents encapsulated within non-permeable macro beads to enable a wider range of delivery vehicles, to provide longer product shelf-life, to allow multiple active (21) Appl. No.: 10/864,149 agents within the composition, to allow the controlled use of the active agents, to provide protected and designable release features and to provide visual inspection for damage (22) Filed: Jun. 9, 2004 and inconsistency. US 2004/0224012 A1 Nov. 11, 2004 TOPCAL APPLICATION AND METHODS FOR 0006 Various limitations on the shelf-life and use of ADMINISTRATION OF ACTIVE AGENTS USING liposome compounds exist due to the relatively fragile LPOSOME MACRO-BEADS nature of liposomes. Major problems encountered during liposome drug Storage in vesicular Suspension are the chemi CROSS REFERENCE TO OTHER cal alterations of the lipoSome compounds, Such as phos APPLICATIONS pholipids, cholesterols, ceramides, leading to potentially toxic degradation of the products, leakage of the drug from 0001) This application claims the benefit of U.S. -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

33-Adrenoceptor-Mediated Relaxation Induced by Isoprenaline And
J. Smooth Muscle Res. 33 : 99-106. 99 The )32 and [33-Adrenoceptor-Mediated Relaxation Induced by Isoprenaline and Salbutamol in Guinea Pig Taenia Caecum Katsuo KOIKE, Tsukasa IcHiNo, Takahiro HORINOUCHI and Issei TAKAYANAGI Departmentof Chemical Pharmacology, Toho University School of PharmaceuticalSciences, 2-2-1, Miyama,Funabashi, Chiba 274, Japan Abstract To understand the receptor subtypes responsible for /3adrenoceptormediated relaxa tion of guinea pig taenia caecum, we investigated the effects of isoprenaline and salbutamol . Isoprenaline and salbutamol caused dose-dependent relaxation of the guinea pig taenia caecum. Propranolol, bupranolol and butoxamine produced shifts of the concentration response curves for isoprenaline and salbutamol. Schild regression analyses carried out for propranolol against isoprenaline and salbutamol gave pA2 values of 8.43 and 8.88, respective ly. Schild regression analyses carried out for butoxamine against isoprenaline and sal butamol gave pA2 values of 6.46 and 6.68, respectively. Schild regression analyses carried out for bupranolol against isoprenaline and salbutamol gave pA2 values of 8.60 and 8.69, respectively. However, in the presence of 3 x 10' M atenolol, 10-4 M butoxamine and 10-6 M phentolamine to block the fir , /32 and a -adrenoceptor effects, respectively, Schild regression analyses carried out for bupranolol against isoprenaline and salbutamol gave pA2 values of 5.77 and 5.97, respectively. These results suggest that the relaxant responses to isoprenaline and salbutamol in the guinea pig taenia caecum are mediated by both the /.32 and the A-adrenoceptors. Key words : f2-adrenoceptor, A-adrenoceptor, isoprenaline, salbutamol , guinea pig taenia caecum Introduction The /3adrenoceptors were subclassified as and /32subtypes based on the agonist potency and tissue localization. -

Examination of Antimicrobial Activity of Selected Non-Antibiotic Medicinal Preparations
Acta Poloniae Pharmaceutica ñ Drug Research, Vol. 69 No. 6 pp.1368ñ1371, 2012 ISSN 0001-6837 Polish Pharmaceutical Society EXAMINATION OF ANTIMICROBIAL ACTIVITY OF SELECTED NON-ANTIBIOTIC MEDICINAL PREPARATIONS HANNA KRUSZEWSKA1*, TOMASZ ZAR BA1 and STEFAN TYSKI1,2 1National Medicines Institute, Department of Antibiotics and Microbiology, 30-34 Che≥mska St., 00-725 Warszawa, Poland 2 Medical University of Warsaw, Department of Pharmaceutical Microbiology, 3 Oczki St., 02-007 Warszawa, Poland Abstract: The aim of this study was to detect and characterize the antimicrobial activity of non-antibiotic drugs, selected from the pharmaceutical products analyzed during the state control performed in National Medicines Institute, Warszawa, Poland. In 2010, over 90 pharmaceutical preparations have been randomly chosen from different groups of drugs. The surveillance study was performed on standard ATCC microbial strains used for drug control: S. aureus, E. coli, P. aeruginosa and C. albicans. It was shown that the drugs listed below inhib- ited growth of at least one of the examined strains: Arketis 20 mg tab. (paroxetine), Buvasodil 150 mg tab. (buflomedile), Halidor 100 mg tab. (bencyclane), Hydroxyzinum espefa 25 mg tab. (hydroxyzine), Norifaz 35 mg tab. (risedronate), Strattera 60 mg cap. (atomoxetine), Tamiflu 75 mg tab. (oseltamivir), Valpro-ratiopharm Chrono 300 mg tab. with longer dissolution (valproate), Vetminth oral paste 24 g+3 g/100 mL (niclozamide, oxybendazol). Strattera cap. showed broad activity spectrum. It inhibited growth of -

Acrolein As a Novel Therapeutic Target for Motor and Sensory Deficits in Spinal Cord Injury
[Downloaded free from http://www.nrronline.org on Wednesday, September 11, 2019, IP: 128.210.106.129] NEURAL REGENERATION RESEARCH April 2014,Volume 9,Issue 7 www.nrronline.org SPECIAL ISSUE Acrolein as a novel therapeutic target for motor and sensory deficits in spinal cord injury Jonghyuck Park1, 2, Breanne Muratori2, Riyi Shi1, 2 1 Department of Basic Medical Sciences, College of Veterinary Medicine, Purdue University, West Lafayette, IN, USA 2 Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, USA Abstract Corresponding author: In the hours to weeks following traumatic spinal cord injuries (SCI), biochemical processes are Riyi Shi, M.D., Ph.D., initiated that further damage the tissue within and surrounding the initial injury site: a process Department of Basic Medical Sciences, termed secondary injury. Acrolein, a highly reactive unsaturated aldehyde, has been shown to play College of Veterinary Medicine, Purdue a major role in the secondary injury by contributing significantly to both motor and sensory defi- University, West Lafayette, IN 47907, cits. In particular, efforts have been made to elucidate the mechanisms of acrolein-mediated dam- USA, [email protected]. age at the cellular level and the resulting paralysis and neuropathic pain. In this review, we will highlight the recent developments in the understanding of the mechanisms of acrolein in motor doi:10.4103/1673-5374.131564 and sensory dysfunction in animal models of SCI. We will also discuss the therapeutic benefits of http://www.nrronline.org/ using acrolein scavengers to attenuate acrolein-mediated neuronal damage following SCI. Accepted: 2014-04-08 Key Words: oxidative stress; spinal cord injury; 3-hydrxypropyl mercapturic acid; acrolein-lysine ad- duct; hydralazine Park J, Muratori B, Shi RY. -
Guidance for the Format and Content of the Protocol of Non-Interventional
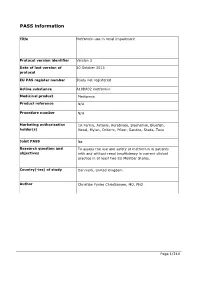
PASS information Title Metformin use in renal impairment Protocol version identifier Version 2 Date of last version of 30 October 2013 protocol EU PAS register number Study not registered Active substance A10BA02 metformin Medicinal product Metformin Product reference N/A Procedure number N/A Marketing authorisation 1A Farma, Actavis, Aurobindo, Biochemie, Bluefish, holder(s) Hexal, Mylan, Orifarm, Pfizer, Sandoz, Stada, Teva Joint PASS No Research question and To assess the use and safety of metformin in patients objectives with and without renal insufficiency in current clinical practice in at least two EU Member States. Country(-ies) of study Denmark, United Kingdom Author Christian Fynbo Christiansen, MD, PhD Page 1/214 Marketing authorisation holder(s) Marketing authorisation N/A holder(s) MAH contact person N/A Page 2/214 1. Table of Contents PASS information .......................................................................................................... 1 Marketing authorisation holder(s) .................................................................................... 2 1. Table of Contents ...................................................................................................... 3 2. List of abbreviations ................................................................................................... 4 3. Responsible parties .................................................................................................... 5 4. Abstract .................................................................................................................. -

(12) United States Patent (10) Patent No.: US 6,784,177 B2 Cohn Et Al
USOO6784177B2 (12) United States Patent (10) Patent No.: US 6,784,177 B2 Cohn et al. (45) Date of Patent: Aug. 31, 2004 (54) METHODS USING HYDRALAZINE Massie et al., The American Journal of Cardiology, COMPOUNDS AND SOSORBIDE 40:794-801 (1977). DINTRATE OR ISOSORBIDE Kaplan et al., Annals of Internal Medicine, 84:639-645 MONONTRATE (1976). Bauer et al., Circulation, 84(1):35-39 (1991). (75) Inventors: Jay N. Cohn, Minneapolis, MN (US); The SOLVD Investigators, The New England Journal of Medicine, 327(10):685–691 (1992). Peter Carson, Chevy Chase, MD (US) Ziesche et al., Circulation, 87(6):VI56-VI64 (1993). Rector et al., Circulation, 87(6):VI71-VI77 (1993). (73) Assignee: Nitro Med, Inc., Bedford, MA (US) Carson et al., Journal of Cardiac Failure, 5(3):178-187 (Sep. 10, 1999). (*) Notice: Subject to any disclaimer, the term of this Dries et al, The New England Journal of Medicine, patent is extended or adjusted under 35 340(8):609-616 (Feb. 25, 1999). U.S.C. 154(b) by 18 days. Freedman et al, Drugs, 54 (Supplement 3):41-50 (1997). Sherman et al., Cardiologia, 42(2):177-187 (1997). (21) Appl. No.: 10/210,113 Biegelson et al., Coronary Artery Disease, 10:241-256 (1999). (22) Filed: Aug. 2, 2002 Rudd et al, Am. J. Physiol., 277(46):H732–H739 (1999). (65) Prior Publication Data Hammerman et al, Am. J. Physiol., 277(46):H1579–1592 (1999). US 2004/0023967 A1 Feb. 5, 2004 LoScalzo et al., Transactions of the American and Climato logical ASS., 111:158-163 (2000). -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

Novel Method of Administering Β-Blockers and Novel Dosage Forms Containing Same Anwar A
University of Kentucky UKnowledge Pharmaceutical Sciences Faculty Patents Pharmaceutical Sciences 1-31-1984 Novel Method of Administering β-Blockers and Novel Dosage Forms Containing Same Anwar A. Hussain University of Kentucky Right click to open a feedback form in a new tab to let us know how this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/ps_patents Part of the Pharmacy and Pharmaceutical Sciences Commons Recommended Citation Hussain, Anwar A., "Novel Method of Administering β-Blockers and Novel Dosage Forms Containing Same" (1984). Pharmaceutical Sciences Faculty Patents. 124. https://uknowledge.uky.edu/ps_patents/124 This Patent is brought to you for free and open access by the Pharmaceutical Sciences at UKnowledge. It has been accepted for inclusion in Pharmaceutical Sciences Faculty Patents by an authorized administrator of UKnowledge. For more information, please contact [email protected]. Unlted States Patent [191 [11] 4,428,883 Hussain [45] Jan. 31, 1984 [54] NOVEL METHOD OF ADMINISTERING 4,012,444 3/l977 Lunts ................................. .. 424/ 324 B-BLOCKERSF0 RMS Co NTA' AND, INING NOVEL SAME DOSAGE ' 4,250,163, , 1.1’;2/l981 llieoll’loldNagaioe a ......................................... ---- -~.. .. 424/14 [7 5 ] I nventor : An war A . Hussam,° Lexington' , Ky . FOREIGN PATENT DOCUMENTS [73] Assignee: The University of Kentucky Research . Foundation’ Lexington, Ky. 979389 1/ 1965 United Klngdom . I [21] APPL Nod 241,413 OTHER PUBLICATIONS . Stern, Arzmit. Forsch, vol. 24, 1974, pp. 70-71. [22] Flled‘ Mm‘- 6’ 1981 Black, Brit. J. PharmacoL, (1965) 25, pp. 577-591. [51] Int. Cl.3 ................... .. A61K 27/00; A61K 31/15; Prima'y Examiner_stanley L Friedman A61K 31/40; A61K 31/47; A61K 31/135; Attorney, Agent, or Firm-Burns, Doane, Swecker & A61K 31/165; A61K 31/435; A61K 31/475; Mathis A61K 47/00; C07C 143/90; CllD 1/28; C09F - 5 /()() [57] ABSTRACT [52] US. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9