Influence of Solvent Extraction, Maturity Stage, and Thermal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Determination of Capsaicinoid Profile of Some Peppers Sold in Nigerian Markets
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(4):648-654 ISSN : 0975-7384 Research Article CODEN(USA) : JCPRC5 Determination of capsaicinoid profile of some peppers sold in Nigerian markets 1N. C. Nwokem *, 2C. O. Nwokem, 2Y. O. Usman, 1O. J. Ocholi, 2M. L. Batari and 3A. A. Osunlaja 1Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria 2National Research Institute for Chemical Technology, Zaria, Nigeria 3Umar Suleiman College of Education, Gashua, Nigeria ____________________________________________________________________________________________ ABSTRACT The capsaicinoid profile of six different peppers sold in Nigerian markets was determined by Gas Chromatography- Mass Spectrometry. The capsaicinoids were extracted from the peppers using methanol as extractant and analyzed without need for derivatization. A total of eight (8) capsaicinoids were identified and quantitated: Capsaicin, Dihydrocapsaicin Dihydrocapsaicin 1, Dihydrocapsaicin 2, Norcapsaicin, Nordihydrocapsaicin 1, Nordihydrocapsaicin 2 and Nornordihydrocapsaicin though, not fully present in all the varieties. Dihydrocapsaicin 1, Dihydrocapsaicin 2, Nordihydrocapsaicin 1, Nordihydrocapsaicin 2 and Nornordihydrocapsaicin are isomers. Seven were identified in the Cameroun pepper variety, six in “Zaria atarugu” and Miango, and five in each of the remaining varieties. In all the peppers analyzed, capsaicin had the highest relative concentration, which ranged from 27.3% in the Cameroun variety to 49.38% in the “Zaria atarugu” variety. The sum of the relative concentrations of capsaicin and dihydrocapsaicin ranged from 47.03% in the “Miango” variety to 87.3% in the “Zaria atarugu” variety. Keywords: Capsaicinoids, Gas Chromatography-Mass Spectrometry, Methanol, Pepper ____________________________________________________________________________________________ INTRODUCTION Peppers are widely used in many parts of the world as a result of their valued sensory attributes; colour, purgency and aroma. -

Determination of Capsaicinoids in Peppers by Microwave-Assisted Extraction–High-Performance Liquid Chromatography with fluorescence Detection Gerardo F
Analytica Chimica Acta 578 (2006) 227–233 Determination of capsaicinoids in peppers by microwave-assisted extraction–high-performance liquid chromatography with fluorescence detection Gerardo F. Barbero, Miguel Palma ∗, Carmelo G. Barroso Grupo de Investigaci´on Qu´ımico Anal´ıtica del Vino y Productos Agroalimentarios, Departamento de Qu´ımica Analitica, Facultad de Ciencias, Universidad de C´adiz, Apartado 40, 11510 Puerto Real, C´adiz, Spain Received 30 March 2006; received in revised form 27 June 2006; accepted 28 June 2006 Available online 4 July 2006 Abstract A new method has been developed for the extraction of capsaicinoids (nordihydrocapsaicin, capsaicin, dihydrocapsaicin, homocapsaicin and homodihydrocapsaicin) in peppers employing microwave-assisted extraction. The parameters studied are: extraction solvent (methanol, ethanol, acetone, ethyl acetate and water), temperature (50–200 ◦C), sample quantity (0.1–1 g), volume of solvent (15–50 mL) and the extraction time (5–20 min). The results found for the optimum conditions are: 125 ◦C as extraction temperature, 25 mL of solvent, 0.5 g of freshly triturated peppers and extraction for 5 min, employing 100% ethanol as solvent. The capsaicinoids obtained were stable under the optimised extraction conditions. The resulting method presents a high degree of reproducibility (R.S.D. < 6%). © 2006 Elsevier B.V. All rights reserved. Keywords: Microwave-assisted extraction; Capsaicinoids; Peppers 1. Introduction Many techniques for the extraction of capsaicinoids from peppers have been studied, such as maceration [9], magnetic stir- Hot or spicy peppers are savoury food additives that are ring [10], Soxhlet [11,12], ultrasound-assisted extraction [13], widely utilised in many parts of the world, and are highly valued extraction by means of supercritical fluids [14], extraction by for their attributes of colour, pungency, and aroma. -

TRP CHANNELS AS THERAPEUTIC TARGETS TRP CHANNELS AS THERAPEUTIC TARGETS from Basic Science to Clinical Use
TRP CHANNELS AS THERAPEUTIC TARGETS TRP CHANNELS AS THERAPEUTIC TARGETS From Basic Science to Clinical Use Edited by ARPAD SZALLASI MD, PHD Department of Pathology, Monmouth Medical Center, Long Branch, NJ, USA AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 125 London Wall, London, EC2Y 5AS, UK 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA 225 Wyman Street, Waltham, MA 02451, USA The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK First published 2015 Copyright © 2015 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangement with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. -

Numex Lotalutein, a Lutein-Rich Serrano Pepper
HORTSCIENCE 55(12):2052–2055. 2020. https://doi.org/10.21273/HORTSCI14949-20 segregating population produced segregating lines of mature fruit that were red, yellow, and orange. Single-plant selection using phe- NuMex LotaLutein, a Lutein-rich notypic recurrent selection with pedigree breeding for earliness, desirable fruit shape Serrano Pepper and size, yield, and ease of destemming was accomplished. A total of seven generations of Ivette Guzman, Danise Coon, Krystal Vargas, and Paul W. Bosland self-pollination were accomplished using a Department of Plant and Environmental Sciences, New Mexico State greenhouse during the winter season. Within University, Las Cruces, NM 88003 the segregating generations, five single-plant selections were made based on the color of Additional index words. Capsicum annuum , carotenoids, high-performance liquid chroma- ripeness alone. Each selected plant was selfed tography, human health, macular degeneration, open-pollinated by stripping fruits and open flowers and then placing an isolation cage over the individual plant to exclude any outcrossing (Bosland, Lutein is a carotenoid with antioxidant modification by the human body (Johnson, 1993). During each generation of selection, and anti-inflammatory properties important 2002). Sources of lutein are green leafy phenotypic traits considered to be impor- for reducing the risks of several chronic vegetables, yellow fruits and vegetables, tant to a serrano pod-type were selected diseases (Buscemi et al., 2018). Biofortifica- and egg yolks (Johnson, 2002). Furthermore, (Bosland and Votava, 2012). In 2013 and tion is the process by which the nutritional many studies have reported that lutein may 2014, selfed, single-plant selections were quality of food crops, like lutein, is improved have positive effects on different clinical planted at the LPSRC, and selections of through plant breeding, genetic medication, conditions, thus ameliorating cognitive func- desired horticultural traits were made and and other agronomic practices. -

Updates and Observations on High Tunnel Production at DSAC
2015 HIGH TUNNEL HEIRLOOM TOMATO & SPECIALTY HOT PEPPER VARIETY TRIALS Shelby Henning Department of Crop Sciences University of Illinois UNIVERSITY OF ILLINOIS RESEARCH AND EDUCATION CENTERS * UNIVERSITY OF ILLINOIS RESEARCH AND EDUCATION * CENTERS * XXXXX XXXXX XXXXXX UNIVERSITY OF ILLINOIS RESEARCH X AND EDUCATION CENTERS XXXXXXXX XXXXXXXXX 2015 HT HEIRLOOM TOMATO TRIAL • Heirloom tomato • Advantages • Flavor • Unique appearance • Profitability • Disadvantages • Tender • Performance issues 2015 HT HEIRLOOM TOMATO TRIAL • Heirloom tomato • Health benefits? • Lycopene - anticancer • Improved transportation & appearance?? • Breaker vs. vine ripe? • HT performance in N IL? www.tasti-lee.com http://www.burpee.com/vegetables/tomatoes/saladette/tomato- health-kick-hybrid-prod000992.html 2015 HT HEIRLOOM TOMATO TRIAL • 22 tomato cultivars Lycopene vs. polyphenols • 20 indeterminate • 2 determinate • 8 Replicates • - 4 analyzed as breakers • - 4 analyzed as vine-ripe • Seeded March 18 • Planted May 9 • Weekly harvests (7/30 – 9/9) 2015 HT TOMATO CULTIVAR TRIAL • Indeterminate • Spaced at 18” in row spacing • Pruned to a single leader • Trained onto single twine • Determinate • Spaced at 18 inches • Pruned to first sucker below first cluster • Trained onto single twine • Fertigated weekly with calcium nitrate and potassium nitrate @ 7 lb 8 oz. N/A TOMATO VARIETIES • Amish Gold Slicer • Gold Medal • Pareso • Arkansas Traveler • Green Zebra • Ponderosa Red • Aunt Ruby's German Green • Health Kick • Sakura • Big beef • Hillbilly Potato Leaf • Stupice • Black Krim • Japanese Black Trifele • Tasti-Lee • Brandywine • Kellog's Breakfast • Cherokee Purple • Mortgage Lifter • Favorita • Nyagous • German Pink 2015 HT TOMATO & BELL PEPPER CULTIVAR TRIAL • Insect Pest Problems • Hornworm • Mosquitos • Disease Problems • No biotic disease that appeared significant July 23, 2015 Sept. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0053319 A1 Perry (43) Pub
US 2009.0053319A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0053319 A1 Perry (43) Pub. Date: Feb. 26, 2009 (54) SORE THROAT RELIEF COMPOSITION AND Publication Classification METHOD OF PRODUCING SAME (51) Int. Cl. A6II 3/165. (2006.01) (76) Inventor: Wye Jeffrey Perry, Albany, NY A6IPA6II 35/64II/00 (2006.01) (52) U.S. Cl. .......................... 424/537; 514/625; 514/627 Correspondence Address: (57)57 ABSTRACT AMIN HALLIHAN, LLC The present invention provides a sore throat relief composi 444 NORTHORLEANS STREET, SUITE 400 tion and a method of producing the composition comprising CHICAGO, IL 60654 (US) oleoresin capsicum containing capsaicin, dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin, and homocap saicin as active ingredients, combined with vegetable glyc (21) Appl. No.: 11/842,458 erin, purified water, spearmint oil, wild cherry bark, clove honey, and ascorbic acid for fully relieving and preventing chronic and occasional Sore throat symptoms, including pain, (22) Filed: Aug. 21, 2007 dryness, and inflammation. US 2009/00533 19 A1 Feb. 26, 2009 SORE THROAT RELEF COMPOSITION AND drocapsaicin is an irritant and has a similar pungency to METHOD OF PRODUCING SAME capsaicin. Nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicinare also irritants and have a pungency of about FIELD OF THE INVENTION 8,600,000-9,100,000 Scoville units. 0001. The present invention relates to a sore throat relief 0003. Each capsaicinoid and its corresponding chemical composition containing natural capsaicinoids for relieving structure is shown below. Capsaicin H O Dihydrocapsaicin O Nordihydrocapsaicin O Homodihydrocapsaicin O N O Homocapsaicin H O Sore throat pain, dryness, and inflammation along with pro 0004 Capsaicinoids are irritants and produce a sensation viding antimicrobial properties. -
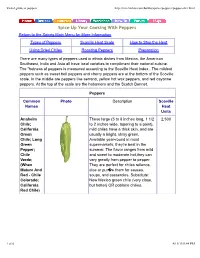
Visual Guide to Peppers
Visual guide to peppers http://missvickie.com/howto/spices/peppers/peppersdict.html Spice Up Your Cooking With Peppers Return to the Spices Main Menu for More Information Types of Peppers Scoville Heat Scale How to Stop the Heat Using Dried Chiles Roasting Peppers Preparation There are many types of peppers used in ethnic dishes from Mexico, the American Southwest, India and Asia all have local varieties to compliment their national cuisine. The "hotness of peppers is measured according to the Scoville Heat Index . The mildest peppers such as sweet bell peppers and cherry peppers are at the bottom of the Scoville scale. In the middle are peppers like serrano, yellow hot wax peppers, and red cayanne peppers. At the top of the scale are the habamero and the Scotch Bonnet. Peppers Common Photo Description Scoville Names Heat Units Anaheim These large (5 to 8 inches long, 1 1/2 2,500 Chile; to 2 inches wide, tapering to a point), California mild chiles have a thick skin, and are Green usually a bright, shiny green. Chile; Long Available year-round in most Green supermarkets, they're best in the Pepper; summer. The flavor ranges from mild Chile and sweet to moderate hot,they can Verde; vary greatly from pepper to pepper. (When They are perfect for chiles rellenos, Mature And dice or pur�e them for sauces, Red - Chile soups, and casseroles. Substitute: Colorado; New Mexico green chile (very close, California but hotter) OR poblano chilies. Red Chile) 1 of 6 4/13/13 9:44 PM Visual guide to peppers http://missvickie.com/howto/spices/peppers/peppersdict.html Banana A banana-shaped pepper that 500 Pepper, changes from pale to deep yellow or Banana orange as they mature. -

Fast Method for Capsaicinoids Analysis from Capsicum Chinense Fruits
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Food Research International 64 (2014) 718–725 Contents lists available at ScienceDirect Food Research International journal homepage: www.elsevier.com/locate/foodres Fast method for capsaicinoids analysis from Capsicum chinense fruits Marla Sganzerla a, Janclei Pereira Coutinho a, Arlete Marchi Tavares de Melo b, Helena Teixeira Godoy a,⁎ a Department of Food Science, Faculty of Food Engineering, University of Campinas (UNICAMP), P.O. Box 6121, 13083-862 Campinas, SP, Brazil b Horticultural Center, Agronomic Institute of Campinas (IAC), P.O. Box 28, 13012-970 Campinas, SP, Brazil article info abstract Article history: Chili peppers are widely utilized in the world as savory food additives due the pungency induced by the Received 28 January 2014 capsaicinoids. Also, these compounds have functional properties as antimutagenic, antitumoral, antioxidant Accepted 14 August 2014 and analgesic. These characteristics increase the interest in this compound class, hence the capsaicinoid analysis Available online 21 August 2014 must be reproducible and accurate. This study aimed to develop and validate a fast, efficient and reproducible method to analyze capsaicinoids in Brazilian Capsicum chinense fruits. The extracts were obtained after an opti- Keywords: Chili pepper mization step that indicated the condition 100% of methanol and 10 min on ultrasound assisted extraction. The Method development analyses were carried out in an ultra high performance liquid chromatographic system with detection by Ultrasound assisted extraction a photo diode array and mass spectrometer. The analytical method developed permits the separation of Ultra high performance liquid chromatography 8 capsaicinoids in 4 min of time analysis expending only 2 mL of solvent as mobile phase. -

Hot Pepper (Capsicum Spp.) – Important Crop on Guam
Food Plant Production June 2017 FPP-05 Hot Pepper (Capsicum spp.) – Important Crop on Guam Joe Tuquero, R. Gerard Chargualaf and Mari Marutani, Cooperative Extension & Outreach College of Natural & Applied Sciences, University of Guam Most Capsicum peppers are known for their spicy heat. Some varieties have little to no spice such as paprika, banana peppers, and bell peppers. The spice heat of Capsicum peppers are measured and reported as Scoville Heat Units (SHU). In 1912, American pharmacist, Wilbur Scoville, developed a test known as the, Scoville Organoleptic Test, which was used to measure pungency (spice heat) of Capsicum peppers. Since the 1980s, pungency has been more accurately measured by high-performance liquid chromatography Source: https://phys.org/news/2009-06-domestication- (HPLC). HPLC tests result in American Spice Trade capsicum-annuum-chile-pepper.html Association (ASTA) pungency units. ASTA pungency Introduction units can be converted to SHU. Table 2 displays Sco- Hot pepper, also known as chili, chilli, or chile pepper, ville Heat Units of various popular Capsicum peppers is a widely cultivated vegetable crop that originates (Wikipedia, 2017). from Central and South America. Hot peppers belong to the genus Capsicum. There are over 20 species under the genus Capsicum. There are five major domesticated species of peppers that are commercially cultivated (Table 1), and there are more than 50,000 varieties. Fig. 1 depicts a unqiue, citrus-flavored variety of Capsicum baccatum hot pepper, known as Lemon Drop (aji-type), popular for seasoning in Peru (Wikipedia, 2017). Table 1. The five major domesticated Capsicum species of pepper with examples of commonly known types of pepper. -

An Alternate Solvent for the Determination of Capsaicin Content in Chillies by HPLC Method
s Chemis ct try u d & o R Srinivasan et al., Nat Prod Chem Res 2018, 6:5 r P e s l e Natural Products Chemistry & a DOI: 10.4172/2329-6836.1000342 r a r u t c h a N Research ISSN: 2329-6836 Research Article Open Access An Alternate Solvent for the Determination of Capsaicin Content in Chillies by HPLC Method Srinivasan GV1*, Davis Thomas Daniel2, Soumya KV3 and Menon KRK4 1Spices Board, Quality Evaluation Laboratory (QEL), Plot No. R-11, SIPCOT Industrial Area, Gummidipoondi, Chennai, Tamil Nadu, India 2Department of Chemistry, St. Stephen’s College, New Delhi-110 007, India 3Spices Board, QEL, Tuticorin-628 004, Tamil Nadu, India 4Spices Board, QEL, Cochin-682 025, Kerala, India *Corresponding author: Srinivasan GV, Scientist C, Spices Board, Quality Evaluation Laboratory (QEL), Plot No. R-11, SIPCOT Industrial Area, Gummidipoondi, Chennai, Tamil Nadu, India, Tel: +(91)-7200313143; E-mail: [email protected] Received: September 01, 2018; Accepted: September 10, 2018; Published: September 18, 2018 Copyright: © 2018 Srinivasan GV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract An alternate solvent namely acetone has been proved effective for the determination of capsaicin content in chillies. Considering its less toxicity and easy to procure compared to methanol or rectified spirit, it can be used as the solvent for the determination of capsaicin content in chillies. The HPLC method adopted for the estimation of capsaicin content has been validated for specificity, linearity, recovery etc. -

'Response to the Director-General's Request
OPCW Scientific Advisory Board Twenty-Fifth Session SAB-25/WP.1 27 – 31 March 2017 27 March 2017 ENGLISH only RESPONSE TO THE DIRECTOR-GENERAL'S REQUEST TO THE SCIENTIFIC ADVISORY BOARD TO PROVIDE CONSIDERATION ON WHICH RIOT CONTROL AGENTS ARE SUBJECT TO DECLARATION UNDER THE CHEMICAL WEAPONS CONVENTION 1. Response to the Director-General’s Request to the Scientific Advisory Board to Consider Which Riot Control Agents are Subject to Declaration Under the Chemical Weapons Convention (hereinafter “the Convention”). Annex: Response to the Director-General’s Request to the Scientific Advisory Board to Consider Which Riot Control Agents are Subject to Declaration Under the Chemical Weapons Convention. CS-2017-0268(E) distributed 27/03/2017 *CS-2017-0268.E* SAB-25/WP.1 Annex page 2 Annex RESPONSE TO THE DIRECTOR-GENERAL’S REQUEST TO THE SCIENTIFIC ADVISORY BOARD TO CONSIDER WHICH RIOT CONTROL AGENTS ARE SUBJECT TO DECLARATION UNDER THE CHEMICAL WEAPONS CONVENTION 1. EXECUTIVE SUMMARY 1.1 This report provides advice from the Scientific Advisory Board (SAB) on which riot control agents (RCAs) would be subject to declaration under the Convention in response to a request by the Director-General at the Board’s Twentieth Session in June 2013 [1]. The request appears in Appendix 1. 1.2 The SAB considered a list of 59 chemicals that included the 14 chemicals declared as RCAs since entry into force of the Convention; chemicals identified as potential RCAs from a list of “riot control agents and old/abandoned chemical weapons” to be considered for inclusion in the OPCW Chemical Agent Database (OCAD) that had been drafted by the SAB’s Temporary Working Group (TWG) on Analytical Procedures in 2001 (Appendix 2) [2]; an initial survey conducted by the Technical Secretariat in 2013 of RCAs that have been researched or are available for purchase, beyond those that are already declared; and 12 additional chemicals recognised by the SAB as having potential RCA applications. -

The Effects of Vanilloid-Like Agents on Platelet Aggregation
THE EFFECTS OF VANILLOID-LIKE AGENTS ON PLATELET AGGREGATION Safa Yousef Almaghrabi, MBBS School of Human Life Sciences Submitted in fulfilment of the requirements for the degree of Master of Biomedical Science (Research) University of Tasmania October 2012 DECLARATION I hereby declare that this thesis entitled The Effects of Vanilloid-Like Agents on Platelet Aggregation contains no material which has been accepted for a degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the my knowledge and belief no material previously published or written by another person except where due reference is made in the text of thesis, nor does the thesis contain any material that infringes copyright. Date: 24th Oct 2012 Signed: AUTHORITY OF ACCESS This thesis may be made available for loan and limited copying and communication in accordance with the Copyright Act 1968. Date: 24th Oct 2012 Signed: STATEMENT OF ETHICAL CONDUCT The research associated with this thesis abides by the international and Australian codes on human and animal experimentation, the guidelines by the Australian Government’s Office of Gene Technology Regulator and the rulings of the Safety, Ethics and Institutional Biosafety Committees of the University. Date: 24th Oct 2012 Signed: Full Name: Safa Yousef O. Almaghrabi i ACKNOWLEDGEMENTS First of all, I would like to thank the Government of Saudi Arabia (King Abdulaziz University) for the scholarship and sponsorship. I would also like to sincerely acknowledge my supervisors, Dr. Murray Adams, A/Prof. Dominic Geraghty, and Dr. Kiran Ahuja for their guidance, tolerance and being there whenever needed.