Experimental Investigation on Performance and Emission
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
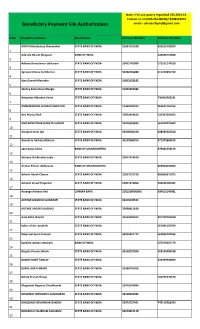
Beneficiary Payment File Authorization Email:- [email protected]
Note:- For any querry regardind CSS 2013-14 Contact us on (020-26126939)/ 8308318919 Beneficiary Payment File Authorization email:- [email protected] S.No Beneficiary Name Bank Name Account Number Aadhaar Number AARTI Mahadeorao Shendurkar STATE BANK OF INDIA 31957537638 659252453204 1 Adamile Bharat Bhagwat BANK OF INDIA 243096723824 2 Adhane Rameshwar Sakharam STATE BANK OF INDIA 33451742999 572551174530 3 Agrawal Antara Sunilkumar STATE BANK OF INDIA 33290256080 311232832732 4 Ajay Ganesh Maturkar STATE BANK OF INDIA 31852633182 5 Akshay Rameshrao Mangle STATE BANK OF INDIA 31904299582 6 Ambarkar Abhishek Vinod STATE BANK OF INDIA 740900562181 7 AMBEWADKAR GOURAV NARAYAN STATE BANK OF INDIA 11365269433 964641761762 8 Ami Manoj Shah STATE BANK OF INDIA 33052414428 226307092832 9 AMILKANTHWAR SHWETA SUDHIR STATE BANK OF INDIA 33415593824 561010421602 10 Aniapure Arun Ajit STATE BANK OF INDIA 33448582440 928583923410 11 Annadate Akshata Mahavir STATE BANK OF INDIA 33135360910 971373889676 12 apar pooja fakira BANK OF MAHARASHTRA 979081533129 13 Archana Shrikrushna Jayle STATE BANK OF INDIA 32047374678 14 Arvikar Pritam Uddhavrao BANK OF MAHARASHTRA 883964656660 15 Ashvini Harish Chavan STATE BANK OF INDIA 31957537729 995866871075 16 Ashwini Vinod Hingankar STATE BANK OF INDIA 32047374850 450533195402 17 Aurange Archana Anil CANARA BANK 2581108006866 999613149981 18 AUTADE MADHURI GANAPATI STATE BANK OF INDIA 33446440532 19 AUTADE SAGAR VILASRAO STATE BANK OF INDIA 33098812108 20 Aute Rohit Sharad STATE BANK OF INDIA 33150350072 -

Top 10 Male Indian Singers
Top 10 Male Indian Singers 001asd Don't agree with the list? Vote for an existing item you think should be ranked higher or if you are a logged in,add a new item for others to vote on or create your own version of this list. Share on facebookShare on twitterShare on emailShare on printShare on gmailShare on stumbleupon4 The Top Ten TheTopTens List Your List 1Sonu Nigam +40Son should be at number 1 +30I feels that I have goten all types of happiness when I listen the songs of sonu nigam. He is my idol and sometimes I think that he is second rafi thumbs upthumbs down +13Die-heart fan... He's the best! Love you Sonu, your an idol. thumbs upthumbs down More comments about Sonu Nigam 2Mohamed Rafi +30Rafi the greatest singer in the whole wide world without doubt. People in the west have seen many T.V. adverts with M. Rafi songs and that is incediable and mind blowing. +21He is the greatest singer ever born in the world. He had a unique voice quality, If God once again tries to create voice like Rafi he wont be able to recreate it because God creates unique things only once and that is Rafi sahab's voice. thumbs upthumbs down +17Rafi is the best, legend of legends can sing any type of song with east, he can surf from high to low with ease. Equally melodious voice, well balanced with right base and high range. His diction is fantastic, you may feel every word. Incomparable. More comments about Mohamed Rafi 3Kumar Sanu +14He holds a Guinness World Record for recording 28 songs in a single day Awards *. -

My Favourite Singers
MY FAVOURITE SINGERS 12 01 01 SAMJHAWAN 17 CHALE CHALO 33 BARSO RE 48 JEENA 63 AY HAIRATHE 79 DIL KYA KARE (SAD) 94 SAAJAN JI GHAR AAYE 108 CHHAP TILAK 123 TUM MILE (ROCK) 138 D SE DANCE ARTIST: ARIJIT SINGH, SHREYA GHOSHAL • ALBUM/FILM: HUMPTY SHARMA KI DULHANIA ARTIST: A. R. RAHMAN, SRINIVAS • ALBUM/FILM: LAGAAN ARTIST: SHREYA GHOSHAL, UDAY MAZUMDAR • ALBUM/FILM: GURU ARTIST: SONU NIGAM, SOWMYA RAOH • ALBUM/FILM: DUM ARTIST: ALKA YAGNIK, HARIHARAN • ALBUM/FILM: GURU ARTIST: KUMAR SANU, ALKA YAGNIK • ALBUM/FILM: DIL KYA KARE ARTIST: KAVITA KRISHNAMURTHY, ALKA YAGNIK, KUMAR SANU ARTIST: KAILASH KHER • ALBUM/FILM: KAILASA JHOOMO RE ARTIST: SHAFQAT AMANAT ALI • ALBUM/FILM: TUM MILE ARTIST: VISHAL DADLANI, SHALMALI KHOLGADE, ANUSHKA MANCHANDA 02 ENNA SONA 18 ROOBAROO 34 KHUSHAMDEED 49 WOH PEHLI BAAR 64 AGAR MAIN KAHOON 80 DEEWANA DIL DEEWANA ALBUM/FILM: KUCH KUCH HOTA HAI 109 CHAANDAN MEIN 124 TERE NAINA ALBUM/FILM: HUMPTY SHARMA KI DULHANIA ARTIST: ARIJIT SINGH • ALBUM/FILM: OK JAANU ARTIST: A. R. RAHMAN, NARESH IYER • ALBUM/FILM: RANG DE BASANTI ARTIST: SHREYA GHOSHAL • ALBUM/FILM: GO GOA GONE ARTIST: SHAAN • ALBUM/FILM: PYAAR MEIN KABHI KABHI ARTIST: ALKA YAGNIK, UDIT NARAYAN • ALBUM/FILM: LAKSHYA ARTIST: UDIT NARAYAN, AMIT KUMAR • ALBUM/FILM: KABHI HAAN KABHI NA 95 MAIN ALBELI ARTIST: KAILASH KHER • ALBUM/FILM: KAILASA CHAANDAN MEIN ARTIST: SHAFQAT AMANAT ALI • ALBUM/FILM: MY NAME IS KHAN 139 VELE 03 GERUA 19 TERE BINA 35 BAHARA 50 TUNE MUJHE PEHCHAANA NAHIN 65 KUCH TO HUA HAI 81 KUCH KUCH HOTA HAI ARTIST: KAVITA KRISHNAMURTHY, SUKHWINDER SINGH • ALBUM/FILM: ZUBEIDAA 110 AA TAYAR HOJA 125 AKHIYAN ARTIST: VISHAL DADLANI, SHEKHAR RAVJIANI • ALBUM/FILM: STUDENT OF THE YEAR ARTIST: ARIJIT SINGH, ANTARA MITRA • ALBUM/FILM: DILWALE ARTIST: A. -

BENGALURU NORTH UNIVERSITY Sri Devaraj Urs Extension, Tamaka, Kolar - 563103 Page No
BENGALURU NORTH UNIVERSITY Sri Devaraj Urs Extension, Tamaka, Kolar - 563103 Page No. : 1 / 23 College : [ 6005 ] Govt. First Grade College, K.G.F Subjectwise I.A. Marks List Degree : [ BSC1 ] - Bachelor of Science IV Semester Examination MAY / JUNE 2020 Subject : D0011 - English, I.A, Max. Marks: 30 Status : FREEZED (READY TO SEND TO EXAMINATION SECTION) Sl.No. Reg. No. Name Marks Remarks 1 S1811201 ABHISHEK S R 030 Thirty Only 2 S1811202 ADARSHA A 029 Twenty Nine Only 3 S1811203 AJAY A 029 Twenty Nine Only 4 S1811204 ANGEL SHILPA SHERINE S 029 Twenty Nine Only 5 S1811205 ARUN A 030 Thirty Only 6 S1811206 AVINASH S 030 Thirty Only 7 S1811207 AYESHA 030 Thirty Only 8 S1811208 CHANDRIKA N 030 Thirty Only 9 S1811209 CHARUMATHI V 030 Thirty Only 10 S1811210 CHITRA C 029 Twenty Nine Only 11 S1811211 DHIVYA L 030 Thirty Only 12 S1811212 DILEEP D 030 Thirty Only 13 S1811213 DIVYA C 030 Thirty Only 14 S1811214 DIVYA K 030 Thirty Only 15 S1811215 JABEZ N R 030 Thirty Only 16 S1811216 JANANEESHWARI V 030 Thirty Only 17 S1811217 JANANI D 030 Thirty Only 18 S1811218 JANAVI SUNDAR RAJ 030 Thirty Only 19 S1811219 JYOTHI R 030 Thirty Only 20 S1811220 KALYANI B 030 Thirty Only 21 S1811221 KARTHIK G 030 Thirty Only 22 S1811222 KISHORE KUMAR S 029 Twenty Nine Only 23 S1811223 KOKILA N 030 Thirty Only 24 S1811224 LATHA S 030 Thirty Only 25 S1811225 LAVANYA R 030 Thirty Only 26 S1811226 LEEKALA POORNA 029 Twenty Nine Only 27 S1811227 LIKITHA M 030 Thirty Only 28 S1811228 LOHITH KUMAR R 030 Thirty Only 29 S1811229 MADHUSHREE K S 030 Thirty Only 30 S1811230 MAGESHWARI K 029 Twenty Nine Only 31 S1811231 MALATHI S 029 Twenty Nine Only Date : 23/07/2020 Entered by Signature of the Teaching Faculty Signature of the HOD Signature of the Principal Absent on this page : 0 Total Absent : 0 IP Address : 117.199.227.136, Date 23/07/2020 BENGALURU NORTH UNIVERSITY Sri Devaraj Urs Extension, Tamaka, Kolar - 563103 Page No. -

Films 2018.Xlsx
List of feature films certified in 2018 Certified Type Of Film Certificate No. Title Language Certificate No. Certificate Date Duration/Le (Video/Digita Producer Name Production House Type ngth l/Celluloid) ARABIC ARABIC WITH 1 LITTLE GANDHI VFL/1/68/2018-MUM 13 June 2018 91.38 Video HOUSE OF FILM - U ENGLISH SUBTITLE Assamese SVF 1 AMAZON ADVENTURE Assamese DIL/2/5/2018-KOL 02 January 2018 140 Digital Ravi Sharma ENTERTAINMENT UA PVT. LTD. TRILOKINATH India Stories Media XHOIXOBOTE 2 Assamese DIL/2/20/2018-MUM 18 January 2018 93.04 Digital CHANDRABHAN & Entertainment Pvt UA DHEMALITE. MALHOTRA Ltd AM TELEVISION 3 LILAR PORA LEILALOI Assamese DIL/2/1/2018-GUW 30 January 2018 97.09 Digital Sanjive Narain UA PVT LTD. A.R. 4 NIJANOR GAAN Assamese DIL/1/1/2018-GUW 12 March 2018 155.1 Digital Haider Alam Azad U INTERNATIONAL Ravindra Singh ANHAD STUDIO 5 RAKTABEEZ Assamese DIL/2/3/2018-GUW 08 May 2018 127.23 Digital UA Rajawat PVT.LTD. ASSAMESE WITH Gopendra Mohan SHIVAM 6 KAANEEN DIL/1/3/2018-GUW 09 May 2018 135 Digital U ENGLISH SUBTITLES Das CREATION Ankita Das 7 TANDAB OF PANDAB Assamese DIL/1/4/2018-GUW 15 May 2018 150.41 Digital Arian Entertainment U Choudhury 8 KRODH Assamese DIL/3/1/2018-GUW 25 May 2018 100.36 Digital Manoj Baishya - A Ajay Vishnu Children's Film 9 HAPPY MOTHER'S DAY Assamese DIL/1/5/2018-GUW 08 June 2018 108.08 Digital U Chavan Society, India Ajay Vishnu Children's Film 10 GILLI GILLI ATTA Assamese DIL/1/6/2018-GUW 08 June 2018 85.17 Digital U Chavan Society, India SEEMA- THE UNTOLD ASSAMESE WITH AM TELEVISION 11 DIL/1/17/2018-GUW 25 June 2018 94.1 Digital Sanjive Narain U STORY ENGLISH SUBTITLES PVT LTD. -
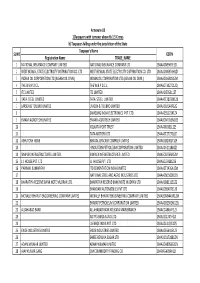
FINAL DISTRIBUTION.Xlsx
Annexure-1B 1)Taxpayers with turnover above Rs 1.5 Crores b) Taxpayers falling under the jurisdiction of the State Taxpayer's Name SL NO GSTIN Registration Name TRADE_NAME 1 NATIONAL INSURANCE COMPANY LIMITED NATIONAL INSURANCE COMPANY LTD 19AAACN9967E1Z0 2 WEST BENGAL STATE ELECTRICITY DISTRIBUTION CO. LTD WEST BENGAL STATE ELECTRICITY DISTRIBUTION CO. LTD 19AAACW6953H1ZX 3 INDIAN OIL CORPORATION LTD.(ASSAM OIL DIVN.) INDIAN OIL CORPORATION LTD.(ASSAM OIL DIVN.) 19AAACI1681G1ZM 4 THE W.B.P.D.C.L. THE W.B.P.D.C.L. 19AABCT3027C1ZQ 5 ITC LIMITED ITC LIMITED 19AAACI5950L1Z7 6 TATA STEEL LIMITED TATA STEEL LIMITED 19AAACT2803M1Z8 7 LARSEN & TOUBRO LIMITED LARSEN & TOUBRO LIMITED 19AAACL0140P1ZG 8 SAMSUNG INDIA ELECTRONICS PVT. LTD. 19AAACS5123K1ZA 9 EMAMI AGROTECH LIMITED EMAMI AGROTECH LIMITED 19AABCN7953M1ZS 10 KOLKATA PORT TRUST 19AAAJK0361L1Z3 11 TATA MOTORS LTD 19AAACT2727Q1ZT 12 ASHUTOSH BOSE BENGAL CRACKER COMPLEX LIMITED 19AAGCB2001F1Z9 13 HINDUSTAN PETROLEUM CORPORATION LIMITED. 19AAACH1118B1Z9 14 SIMPLEX INFRASTRUCTURES LIMITED. SIMPLEX INFRASTRUCTURES LIMITED. 19AAECS0765R1ZM 15 J.J. HOUSE PVT. LTD J.J. HOUSE PVT. LTD 19AABCJ5928J2Z6 16 PARIMAL KUMAR RAY ITD CEMENTATION INDIA LIMITED 19AAACT1426A1ZW 17 NATIONAL STEEL AND AGRO INDUSTRIES LTD 19AAACN1500B1Z9 18 BHARATIYA RESERVE BANK NOTE MUDRAN LTD. BHARATIYA RESERVE BANK NOTE MUDRAN LTD. 19AAACB8111E1Z2 19 BHANDARI AUTOMOBILES PVT LTD 19AABCB5407E1Z0 20 MCNALLY BHARAT ENGGINEERING COMPANY LIMITED MCNALLY BHARAT ENGGINEERING COMPANY LIMITED 19AABCM9443R1ZM 21 BHARAT PETROLEUM CORPORATION LIMITED 19AAACB2902M1ZQ 22 ALLAHABAD BANK ALLAHABAD BANK KOLKATA MAIN BRANCH 19AACCA8464F1ZJ 23 ADITYA BIRLA NUVO LTD. 19AAACI1747H1ZL 24 LAFARGE INDIA PVT. LTD. 19AAACL4159L1Z5 25 EXIDE INDUSTRIES LIMITED EXIDE INDUSTRIES LIMITED 19AAACE6641E1ZS 26 SHREE RENUKA SUGAR LTD. 19AADCS1728B1ZN 27 ADANI WILMAR LIMITED ADANI WILMAR LIMITED 19AABCA8056G1ZM 28 AJAY KUMAR GARG OM COMMODITY TRADING CO. -
![[01900000-02050000] (702Kb)](https://docslib.b-cdn.net/cover/1802/01900000-02050000-702kb-4661802.webp)
[01900000-02050000] (702Kb)
Dated : 23/4/2016 Signatory ID Name CIN Company Name Defaulting Year 01900025 KHATOD PUKHRAJ U17119GJ1993PTC020009 ALANKAR TEXTURISING 2008-09, 2009-10 SANTOSHDEVI PVT LTD 01900054 MANAGARAJ U17111TZ2006PTC013310 KEERTHANA TEXTILES 2008-09, 2009-10 MURUGASAMY MILLS PRIVATE LIMITED 01900137 KENNEDY MUTHIAH U15400TN2008PTC067049 GAYATHRI FROZEN 2008-09, 2009-10 VICTORJOHN PRODUCTS PRIVATE 01900159 PANKAJ SABOO U25209DL2001PTC112780 QUALITY FOAM 2007-08, 2008-09, 2009-10 PROCESSORS PRIVATE 01900231 SHEIKH ZAHIR U52590MP2007PTC020067 AIM INDIA MARKETING 2008-09, 2009-10 PRIVATE LIMITED 01900257 HUSSAIN NADEEM U52590MP2007PTC020067 AIM INDIA MARKETING 2008-09, 2009-10 PRIVATE LIMITED 01900302 DEVI MAHAWAR VIDYA U67120CT2006PTC020073 GIGANTIC CAPITAL PRIVATE 2009-10 LIMITED 01900306 MANIVANNAN PRAKASH U51505TN2007PTC065588 ROYAL REF - TECH PRIVATE 2008-09, 2009-10 LIMITED 01900309 APELAGUNTA VINIL U93000TG2008PTC057437 ARROSE ADVISORY 2008-09, 2009-10 SERVICES PRIVATE LIMITED 01900314 MAHAWAR SAPNA U67120CT2006PTC020073 GIGANTIC CAPITAL PRIVATE 2009-10 LIMITED 01900317 RAVIKUMARARAJAA U20211TN1998PLC041178 SUPER PANEL LIMITED 2007-08, 2008-09, 2009-10 SINGARAVELU VINAYAGA 01900326 JATINDER KUMAR U45201PB2007PTC031492 ORA INFRASTRUCTURE 2008-09, 2009-10 PRIVATE LIMITED 01900332 KAMALEE RAJA U20211TN1998PLC041178 SUPER PANEL LIMITED 2007-08, 2008-09, 2009-10 01900343 GUPTA KSHITIJ U45400DL2008PTC172673 KVG ESTATE PRIVATE 2009-10 LIMITED 01900355 ARUN SINGARAVELU U20211TN1998PLC041178 SUPER PANEL LIMITED 2007-08, 2008-09 VINAYAGA 01900357 ALLETE VENKATRAM U51900TG2007PTC056860 THIRD EYE RECONN 2008-09, 2009-10 PRIVATE LIMITED 01900369 LAKSHMI MIKKILINENI SREE U74999TG2008PTC058270 ESSVI ENTERPRISES (INDIA) 2008-09, 2009-10 PRIVATE LIMITED 01900381 SHAUN SWEENEY U74140DL2008PTC182850 EAST LINK CONSULTING 2009-10 INDIA PRIVATE LIMITED. 01900415 FLAHERTY PAUL THOMAS U74140DL2008PTC182850 EAST LINK CONSULTING 2009-10 INDIA PRIVATE LIMITED. 01900431 ALI MUNNAWAR U18101DL2008ULT172186 SHAHNAI APPARELS 2009-10 PRIVATE LIMITED 01900457 ASHOK KUMAR U29308DL1997PTC086966 R.S. -

Hospital & Clinic List.Xlsx
Sr.no Name of Private Practitioners Qualification Address Mobile no Degree / Diploma 1 Dr shwetali Mhatugade BAMS Rabadagaon 9967419409 2 Dr.Avinesh Dhande BHMS Rabadagaon 7768901805 3 Dr.Sharika Patvekar BAMS Sec.8A Ganesh darshan Shop no.5 Airoli 9769091447 4 Dr.Makrand Nikumbh BHMS Rabadagaon 9987908555 5 Dr.Anuradha Das BHMS Sec.8 Airoli 9820183922 6 Dr.Mohan Gulhane BAMS Rabadanaka Shopline 9820566860 7 Dr.Reshma Shivtare BAMS Sec.8A Airoli 9881315708 8 Dr.Shailesh Shetty MBBS Sec 8A Edan Soc. 9324439603 9 Dr.Gauri Singi BDS Sec.16 Airoli 10 Dr.Uma Wagle PGDHA Sec.8A Airoli Yash Paradise 9967585101 11 Dr.Sangeeta Jadhav BAMS Sec.8A Airoli Yash Paradise 9930950365 12 Dr Girish Shinde BAMS Sec.8A Airoli Soham Soc. 9004279040 13 Dr Shushma Malpani MBBS Sec.8 Airoli Someshwar Tower1st floor plot 9821140226 no.50 14 Dr.chandrakant Dhande BAMS Divagaon near Vithalmandir 15 Dr.Sunil kharde GECH Sec.17 Airoli 9967565324 16 Dr.Arun Avti BAMS AL1/475 Shivshankar apt.Sec.16 Airoli 9821024215 17 Dr.Kriti Bhimbar BHMS Ramanand Nivas Sec.9 Divagaon Airoli 9967705484 18 Dr.Chandrakant Salunke LECH Sec.9 Airoli 9221964014 19 Dr,Rupali Mendhe BHMS Sahjivan AL4/1-3 Sec.16 Airoli 9820098915 20 Dr.Ashavari Deshpande MBBS DCH A-25 Darshan Society Sec.15 Airoli 9820208703 21 Dr.Vasundhara Bendale MBBS Asthbhuja Soc.Sec.5 Airoli 9820866046 22 Dr.Sonali Mandal MD Sec.5 jayjawan Apt. 9833180915 23 Dr.Ravindra Taori MBBs Panchdeep Apt.bld no.1 Sec.17 Airoli 27790607 24 Dr.Pratik Koli Obs.&gnac Sidhivinayak Apt.Sec.5 Airoli 9820095623 BDS 25 Dr.Swati Mokashi MBBS Sec.9 Divagaon 26 Dr.Rajesh Yadav Sec.9 Divagaon 9967786546 27 Dr.Nirmala Pinto MBBS Sec5 Airoli Balaji Soc. -

S. No. INSTITUTE NAME STATE LAST NAME FIRST NAME PROGRAMME COURSE 1 SIDDAGANGA INSTITUTE of TECHNOLOGY Karnataka P EVLIN VIDYULA
S. No. INSTITUTE NAME STATE LAST NAME FIRST NAME PROGRAMME COURSE 1 SIDDAGANGA INSTITUTE OF Karnataka P EVLIN ENGINEERING AND COMPUTER SCIENCE & ENGINEERING TECHNOLOGY VIDYULATHA TECHNOLOGY 2 SIDDAGANGA INSTITUTE OF Karnataka S. RAMU ENGINEERING AND INFORMATION SCIENCE AND TECHNOLOGY TECHNOLOGY ENGINEERING 3 SIDDAGANGA INSTITUTE OF Karnataka C.T NIRANJAN ENGINEERING AND COMPUTER SCIENCE & ENGINEERING TECHNOLOGY TECHNOLOGY 4 SIDDAGANGA INSTITUTE OF Karnataka B.A SRIDHARA ENGINEERING AND ELECTRICAL AND ELECTRONICS TECHNOLOGY TECHNOLOGY ENGINEERING 5 SIDDAGANGA INSTITUTE OF Karnataka S VIRUPAXI ENGINEERING AND MANUFACTURING SCIENCE AND TECHNOLOGY AURADI TECHNOLOGY ENGINEERING 6 SIDDAGANGA INSTITUTE OF Karnataka M G GAUTHAM ENGINEERING AND MECHANICAL ENGINEERING TECHNOLOGY TECHNOLOGY 7 SIDDAGANGA INSTITUTE OF Karnataka K.M MALLESH ENGINEERING AND TRANSPORTATION ENGINEERING AND TECHNOLOGY TECHNOLOGY MANAGEMENT 8 SIDDAGANGA INSTITUTE OF Karnataka N.S VENKATESH ENGINEERING AND MECHANICAL ENGINEERING TECHNOLOGY GUPTA TECHNOLOGY 9 SIDDAGANGA INSTITUTE OF Karnataka DESAI GIRISH ENGINEERING AND CHEMICAL ENGINEERING TECHNOLOGY TECHNOLOGY 10 SIDDAGANGA INSTITUTE OF Karnataka G.V PRABHUSHAN ENGINEERING AND INDUSTRIAL ENGINEERING AND TECHNOLOGY KAR TECHNOLOGY MANAGEMENT 11 BNM INSTITUTE OF TECHNOLOGY Karnataka J REGINA ENGINEERING AND COMPUTER SCIENCE AND TECHNOLOGY ENGINEERING 12 BNM INSTITUTE OF TECHNOLOGY Karnataka H.R. DEEPA ENGINEERING AND ELECTRONICS & COMMUNICATION TECHNOLOGY ENGG 13 BNM INSTITUTE OF TECHNOLOGY Karnataka SURESH PRIYASHREE ENGINEERING -

SASTRA UNIVERSITY Tirumalaisamudram, Thanjavur - 613401 CBCS Registration Key List - (2010 - 11)
SASTRA UNIVERSITY Tirumalaisamudram, Thanjavur - 613401 CBCS Registration Key List - (2010 - 11) Program : B.Tech. - Electronics & Communication Engineering Sem : 4 Sec : A Sl.No. Register No./ Student Name Student ID 1113004006 Achuthaan, A 2 113004011 Aishwarya, S 3 113004022 Archana, R 4113004025 Arun, R 5113004039 Bharath, N 6113004047 Bussi Reddy Raghavadeep Reddy 7113004058 Dharan, S 8113004062 Dinesh, J 9113004068 Eswer, K 10 113004075 Geetha, R 11113004079 Gurrala Ajaykumar 12113004093 Karthikeyan [10/11/91], K 13 113004096 Konda Chandra Harika 14113004100 Krithivasan, H 15113004107 Malolan, S 16 113004113 Meghana Sruthi Pydipati 17113004120 Nangnoori Chandra Kanth 18113004121 Naredla Abhilash Reddy 19113004123 Navin Barathwaj, MA 20 113004124 Nichenametla Hareeka 21 113004126 Nivedha, S 22 113004128 Pagadala Ramya Rekha Reddy 23 113004130 Parvathavarthini, R 24113004136 Pranav, R 25113004142 Prithvi Raj, A 26113004145 Raghuraman, S 27 113004168 Savitha, C 28 113004170 Selva Brindha, M 29 113004171 Shalini, R K 30113004173 Shiva Chidambaram, V A 31 113004182 Soundarya, M 24/12/2010 6.08 PM Page 1 of 10 SASTRA UNIVERSITY Tirumalaisamudram, Thanjavur - 613401 CBCS Registration Key List - (2010 - 11) Program : B.Tech. - Electronics & Communication Engineering Sem : 4 Sec : A Sl.No. Register No./ Student Name Student ID 32113004190 Srivathsava, A K 33113004198 Sureshkumar, K 34 113004204 Swetha, V 35113004205 Takkoli Sivanarayana Reddy 36 113004210 Vaishnavi Ganesh 37 113004211 Vaishnavi, S 38113004225 Vignesh, T 39113004226 Vigneshwar, -

AR Rahman Raanjhanaa Mp3, Flac
A.R. Rahman Raanjhanaa mp3, flac, wma DOWNLOAD LINKS (Clickable) Genre: Pop / Folk, World, & Country / Stage & Screen Album: Raanjhanaa Country: India Released: 2013 Style: Bollywood, Soundtrack MP3 version RAR size: 1308 mb FLAC version RAR size: 1440 mb WMA version RAR size: 1402 mb Rating: 4.1 Votes: 962 Other Formats: WMA AA MP1 MP3 FLAC ASF AUD Tracklist Hide Credits 1 –Shiraz Uppal & Jaswinder Singh Raanjhanaa Banarasiya 2 –Shreya Ghoshal Backing Vocals – Anweshaa, Meenal Jain 3 –Sukhwinder Singh & KMMC Sufi Ensemble Piya Milenge 4 –Madhushree, Chinmayi, Vaishali, Aanchal Sethi Ay Sakhi Nazar Laaye 5 –Rashid Ali, Neeti Mohan Backing Vocals – Nakash Aziz 6 –A.R. Rahman, Rabbi Tu Mun Shudi Aise Na Dekho 7 –A.R. Rahman Backing Vocals – Karthik 8 –Unknown Artist The Land Of Shiva B5 –Javed Ali, Keerthi Sagathia, Pooja Tum Tak Companies, etc. Manufactured By – Sony DADC Manufacturing India Pvt. Ltd. Licensed From – Sony Music Entertainment India Pvt. Ltd. Licensed To – Sony DADC Marketed By – Sony DADC Manufacturing India Pvt. Ltd. Distributed By – Sony DADC Manufacturing India Pvt. Ltd. Copyright (c) – Sony Music Entertainment India Pvt. Ltd. Phonographic Copyright (p) – Sony Music Entertainment India Pvt. Ltd. Credits Lyrics By – Irshad Kamil Music By – A.R. Rahman Notes Eros International Presents- "Raanjhanaa" A Film by Aanand L Rai An Eros International Worldwide Release © & ℗ 2013 Sony Music Entertainment India Pvt. Ltd. Barcode and Other Identifiers Barcode: 8 88837 44672 3 Other: Pkd. 06/2013 Other versions Category Artist Title (Format) Label Category Country Year Sony 88883 74467 2, A.R. Raanjhanaa Music, 88883 74467 2, India 2013 88883 74788 2 Rahman (4xCD) Sony 88883 74788 2 Music A.R. -

Latest Song Volume - 8 a Collection of Latest Hindi Movies Songs
Latest Song Volume - 8 A collection of Latest Hindi Movies songs. Total 321 Hindi songs including Hits of 2016. For detail of Song, TM Song Number, Composer, Movie check the 321 songs list SONG CARD in Specification Ae Dil Hai Mushkil Love You Zindagi Volume Ae Zindagi Gale Laga Le OK Jaanu Bulleya The Breakup Song 8 Channa Mereya The Humma Song Enna Sona Tu Hi Hai Jee Lein Phone: +91 0265 2255704/05 .+91 9904000289 Help Line No.: +91 8511133142 Latest Songs Volume-8 321 Songs No Songs Name Singer Movie Songs In 13309 Aa Re Preetam Pyaare Sarosh Mamta Sharma Rowdy Rathore Vol-8 13310 Aaj Hai Sagaai Abhijeet Alkaji Pyaar To Hona Hi Tha Vol-8 13311 Aala Re Aala Sunidhi Mika Singh Shootout At Wadala Vol-8 13312 Aana Mere Pyar Ko Alkaji Kumar Sanu Kabhi Haan Kabhi Naa Vol-8 13313 Aankhen Bandh Karke Uditji Alkaji Aitraaz Vol-8 13314 Aankhon Mein Jal Raha Hai KyunJagjit Singh Marasim Vol-8 13315 Aao Na Aao Na Jatin Pandit Phir Bhi Dil Hai Hindustani Vol-8 13316 Aar Dariya Sukhwinder Kirti 3 Thay Bhai Vol-8 13317 Aashiq Hoon Main Ashaji Uditji Pyaar To Hona Hi Tha Vol-8 13318 Aashiyan Shreya Ghoshal Nikhil Paul GeorgeBarfi Vol-8 13319 Abhi Mujh Mein Kahin Sonuji Agneepath Vol-8 13320 Achha Lagta Hai Shankar Ehsaan Loy Aarakshan Vol-8 14332 Ae Dil Hai Mushkil Arijit Singh Ae Dil Hai Mushkil Vol-8 14333 Ae Zindagi Gale Laga Le Arijit Singh Dear Zindagi Vol-8 13321 Aetbaar Abhijeet Alkaji Aetbaar Vol-8 13322 Agar Main Kahoon Alkaji Uditji Lakshya Vol-8 13323 Aise Na Dekho A R Rahman Karthik Raanjhanaa Vol-8 13324 Aitraaz Female Sunidhi Aitraaz