UC Berkeley UC Berkeley Electronic Theses and Dissertations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Engine Components and Filters: Damage Profiles, Probable Causes and Prevention
ENGINE COMPONENTS AND FILTERS: DAMAGE PROFILES, PROBABLE CAUSES AND PREVENTION Technical Information AFTERMARKET Contents 1 Introduction 5 2 General topics 6 2.1 Engine wear caused by contamination 6 2.2 Fuel flooding 8 2.3 Hydraulic lock 10 2.4 Increased oil consumption 12 3 Top of the piston and piston ring belt 14 3.1 Hole burned through the top of the piston in gasoline and diesel engines 14 3.2 Melting at the top of the piston and the top land of a gasoline engine 16 3.3 Melting at the top of the piston and the top land of a diesel engine 18 3.4 Broken piston ring lands 20 3.5 Valve impacts at the top of the piston and piston hammering at the cylinder head 22 3.6 Cracks in the top of the piston 24 4 Piston skirt 26 4.1 Piston seizure on the thrust and opposite side (piston skirt area only) 26 4.2 Piston seizure on one side of the piston skirt 27 4.3 Diagonal piston seizure next to the pin bore 28 4.4 Asymmetrical wear pattern on the piston skirt 30 4.5 Piston seizure in the lower piston skirt area only 31 4.6 Heavy wear at the piston skirt with a rough, matte surface 32 4.7 Wear marks on one side of the piston skirt 33 5 Support – piston pin bushing 34 5.1 Seizure in the pin bore 34 5.2 Cratered piston wall in the pin boss area 35 6 Piston rings 36 6.1 Piston rings with burn marks and seizure marks on the 36 piston skirt 6.2 Damage to the ring belt due to fractured piston rings 37 6.3 Heavy wear of the piston ring grooves and piston rings 38 6.4 Heavy radial wear of the piston rings 39 7 Cylinder liners 40 7.1 Pitting on the outer -

UNIVERSAL-- AMERICAN LEADER in MARINE POWER Slnce 1898
MANUAL NO. 1-89 ---UNIVERSAL-- AMERICAN LEADER IN MARINE POWER SlNCE 1898 SERVICE MANUAL MODELS M-12 M2-t2 M3-20 M4-30 M-18 M-25 M-25XP M-35 -------[IDID]]~o~I----- MANUAL NO. 1-89 ---UNIVERSAL-- AMERICAN LEADER IN MARINE POWER SlNCE 1898 SERVICE MANUAL MODELS M-12 M2-t2 M3-20 M4-30 M-18 M-25 M-25XP M-35 -------[IDID]]~o~I----- CONTENTS NOTE: Refer to the beginning of the individual sections for a complete table of contents for that section. SECTION I - SPECIFICATIONS .................................................... 1-38 SECTION II - PREVENTIVE MAINTENANCE ........................................ 39-44 SECTION III - CONSTRUCTION AND FUNCTION .................................... 45-50 SECTION IV - LUBRICATION, COOLING, AND FUEL SYSTEMS ....................... 51-69 SECTION V - ELECTRICAL SYSTEM ............................................. 71-102 SECTION VI - DISASSEMBLY AND REASSEMBLy ................................ 103-150 SECTION VII - DYNAMO AND REGULATOR ...................................... 151-165 TROUBLESHOOTING ......................................................... 166-175 NOTES ii SECTION I - SPECIFICATIONS Model M-12 ..................................................................... 2,3 Model M2-12 .................................................................... 4,5 Model M3-20 . 6,7 Model M4-30 .................................................................... 8,9 Model M-18 ................................................................... 10,11 Model M-25 .................................................................. -

Piston and Connecting Rod Assembly January 2013
1 50-13 1 1 50-13 SUBJECT DATE Installation of the Piston and Connecting Rod Assembly January 2013 Additions, Revisions, or Updates Publication Number / Title Platform Section Title Change DDC-SVC-MAN-0081 Piston and Connecting DD Platform Added special tool chart. Altered wording in step 21. Rod Assembly All information subject to change without notice. 3 1 50-13 Copyright © 2013 DETROIT DIESEL CORPORATION 2 Installation of the Piston and Connecting Rod Assembly 2 Installation of the Piston and Connecting Rod Assembly Table 1. Service Tools Used in the Procedure Tool Number Description W470589011400 DD13 Carbon Scraper Ring tool W470589021400 DD15 Carbon Scraper Ring tool W470589005900 DD13 Piston Ring Compressor J-47386 DD15/16 Piston Ring Compressor W470589015900 DD15/16 Piston Ring Compressor W470589002500 DD15/16 Cylinder Head Leak Tool NOTICE: DO NOT over-expand the piston rings. Over expansion of the piston rings during installation can lead to hairline cracks resulting in ring failure. Install as follows: 1. If the rings have been removed, install them into the grooves of the piston and rotate 120° apart as follows: a. Install the oil ring expander in the lowest groove in the piston. b. Install the oil control ring (top label up) in the lowest groove around the oil ring expander. c. Install the compression ring (top label up) in the middle groove. d. Install the fire ring (top label up) in the top groove. 2. Allowable new ring end gaps for (A), (B), and (C) are shown below. 4 All information subject to change without notice. Copyright © 2013 DETROIT DIESEL CORPORATION 1 50-13 1 50-13 Table 2. -

1 Fuels and Combustion Bengt Johansson
1 1 Fuels and Combustion Bengt Johansson 1.1 Introduction All internal combustion engines use fuel as the source for heat driving the thermo- dynamic process that will eventually yield mechanical power. The fuel properties are crucial for the combustion process. Some combustion processes require a fuel that is very prone to ignition, and some have just the opposite requirement. Often, there is a discussion on what is the optimum. This optimum can be based on the fuel or the combustion process. We can formulate two questions: • What is the best possible fuel for combustion process x? • What is the best possible combustion process for fuel y? Both questions are relevant and deserve some discussion, but it is very seldom that the fuel can be selected without any considerations, and similarly, there is only a limited selection of combustion processes to choose from. This brief intro- duction discusses the combustion processes and the link to the fuel properties that are suitable for them. Thus, it is more in the line of the first question ofthe aforementioned two. 1.2 The Options For internal combustion engines, there are three major combustion processes: • Spark ignition (SI) with premixed flame propagation • Compression ignition (CI) with nonpremixed (diffusion) flame • Homogeneous charge compression ignition, HCCI with bulk autoignition of a premixed charge. These three processes can be expressed as the corner points in a triangle accord- ing to Figure 1.1. Within this triangle, all practical concepts reside. Some are a combination of SI and HCCI, some a combination of SI and CI, and others a Biofuels from Lignocellulosic Biomass: Innovations beyond Bioethanol, First Edition. -

The Influence of Lubricant Degradation on Measured Piston Ring Film Thickness in a Fired Gasoline Reciprocating Engine
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Bradford Scholars The Influence of Lubricant Degradation on Measured Piston Ring Film Thickness in a Fired Gasoline Reciprocating Engine Rai Singh Notay1), Martin Priest2) and Malcolm F Fox2) 1) [email protected] 2) Faculty of Engineering and Informatics, University of Bradford, Bradford, BD7 1DP, UK Abstract A laser induced fluorescence system has been developed to visualise the oil film thickness between the piston ring and cylinder wall of a fired gasoline engine via a small optical window mounted in the cylinder wall. A fluorescent dye was added to the lubricant in the sump to allow the lubricant to fluoresce when absorbing laser radiation. The concentration of the dye did not disturb the lubricant chemistry or its performance. Degraded engine oil samples were used to investigate the influence of lubricant quality on ring pack lubricant film thickness measurements. The results show significant differences in the lubricant film thickness profiles for the ring pack when the lubricant degrades which will affect ring pack friction and ultimately fuel economy. 1. Introduction With the drive towards better energy resource utilisation and an improved environment, current automotive engine tribology research is geared towards reduced pollutant emissions and improved efficiency. A large proportion of the internal friction of an engine is due to the piston assembly, comprising the piston ring pack and the piston skirt, and the lubricant in the ring pack also plays a vital role in exhaust emissions control [1, 2]. Demands on the engine lubricant to help improve engine efficiency are becoming more intense and recent engine technology, such as engine downsizing and stop start functions, are increasing the stress on modern engine lubricants. -

Service Manual
CH18-CH25, CH620-CH730, CH740, CH750 Service Manual IMPORTANT: Read all safety precautions and instructions carefully before operating equipment. Refer to operating instruction of equipment that this engine powers. Ensure engine is stopped and level before performing any maintenance or service. 2 Safety 3 Maintenance 5 Specifi cations 14 Tools and Aids 17 Troubleshooting 21 Air Cleaner/Intake 22 Fuel System 28 Governor System 30 Lubrication System 32 Electrical System 48 Starter System 57 Clutch 59 Disassembly/Inspection and Service 72 Reassembly 24 690 06 Rev. C KohlerEngines.com 1 Safety SAFETY PRECAUTIONS WARNING: A hazard that could result in death, serious injury, or substantial property damage. CAUTION: A hazard that could result in minor personal injury or property damage. NOTE: is used to notify people of important installation, operation, or maintenance information. WARNING WARNING CAUTION Explosive Fuel can cause Accidental Starts can Electrical Shock can fi res and severe burns. cause severe injury or cause injury. Do not fi ll fuel tank while death. Do not touch wires while engine is hot or running. Disconnect and ground engine is running. Gasoline is extremely fl ammable spark plug lead(s) before and its vapors can explode if servicing. CAUTION ignited. Store gasoline only in approved containers, in well Before working on engine or Damaging Crankshaft ventilated, unoccupied buildings, equipment, disable engine as and Flywheel can cause away from sparks or fl ames. follows: 1) Disconnect spark plug personal injury. Spilled fuel could ignite if it comes lead(s). 2) Disconnect negative (–) in contact with hot parts or sparks battery cable from battery. -

Service Bulletin 13-081
Service Bulletin 13-081 May 18, 2016 50065-02166 Version 10 Warranty Extension: MIL Comes On With DTCs P0301 thru P0304 Supersedes 13-081, dated November 24, 2015, to revise the information highlighted in yellow. AFFECTED VEHICLES Year Model Trim VIN Range 2011–13 Odyssey ALL Check the iN VIN Status for Eligibility REVISON SUMMARY • Under REPAIR PROCEDURE B – PISTON CLEANING AND RING REPLACEMENT, Replace Parts as Needed for Repair Procedure B, changed bolt count for the 12 x 163 Washer-Bolt (P/N 90005-PAA-A01) under Cyl 1-4 from 8 to 16. Also changed the bolt count for Connecting Rod Bolt from 8 to 6. CORRECTIVE ACTION 2011 Models: Complete diagnosis procedure and if necessary, update PCM software (Procedure A), replace affected spark plugs then clean pistons and replace the piston rings (Procedure B). 2012–13 Models: Complete diagnosis procedure, replace affected spark plugs then clean the pistons and replace the pistons rings (Procedure B). No need for software update as 2012–13 model vehicles are equipped with updated software from factory. BACKGROUND American Honda is announcing a powertrain warranty extension as a result of a settlement of a class action captioned, Soto et al.v. American Honda Motor Co., Inc., Case No. 3:12-cv-1377-SI (N.D. Cal.). The piston rings on certain cylinders may rotate and align, which can lead to spark plug fouling. This can set DTCs P0301 No. 1 cylinder misfire detected, P0302 No. 2 cylinder misfire detected, P0303 No. 3 cylinder misfire detected, P0304 No. 4 cylinder misfire detected, and cause the MIL to come on. -

Experimental Study on the Effects of Spray-Wall Interaction on Partially Premixed Combustion (PPC) and Engine Emissions
Experimental study on the effects of spray- wall interaction on partially premixed combustion (PPC) and engine emissions Item Type Article Authors Tang, Qinglong; An, Yanzhao; Raman, Vallinayagam; Shi, Hao; Sim, Jaeheon; Chang, Junseok; Magnotti, Gaetano; Johansson, Bengt Citation Tang Q, An Y, Raman V, Shi H, Sim J, et al. (2019) Experimental study on the effects of spray-wall interaction on partially premixed combustion (PPC) and engine emissions. Energy & Fuels. Available: http://dx.doi.org/10.1021/ acs.energyfuels.9b00602. Eprint version Post-print DOI 10.1021/acs.energyfuels.9b00602 Publisher American Chemical Society (ACS) Journal Energy & Fuels Rights This document is the Accepted Manuscript version of a Published Work that appeared in final form in Energy & Fuels, copyright © American Chemical Society after peer review and technical editing by the publisher. To access the final edited and published work see https://pubs.acs.org/doi/pdf/10.1021/ acs.energyfuels.9b00602. Download date 24/09/2021 19:52:45 Link to Item http://hdl.handle.net/10754/652851 Subscriber access provided by King Abdullah University of Science and Technology Library Combustion Experimental study on the effects of spray-wall interaction on partially premixed combustion (PPC) and engine emissions Qinglong Tang, Yanzhao An, Vallinayagam Raman, Hao Shi, Jaeheon Sim, Junseok Chang, Gaetano Magnotti, and Bengt Johansson Energy Fuels, Just Accepted Manuscript • DOI: 10.1021/acs.energyfuels.9b00602 • Publication Date (Web): 01 May 2019 Downloaded from http://pubs.acs.org on May 8, 2019 Just Accepted “Just Accepted” manuscripts have been peer-reviewed and accepted for publication. They are posted online prior to technical editing, formatting for publication and author proofing. -

Piston Rings for Combustion Engines
KNOWLEDGEPOOL PISTON RINGS FOR COMBUSTION ENGINES OUR HEART BEATS FOR YOUR ENGINE. MOTORSERVICE GROUP QUALITY AND SERVICE FROM A SINGLE SOURCE The Motorservice Group is the sales organisation for the worldwide aftermarket activities of Rheinmetall Automotive. It is a leading supplier of engine components for the inde- pendent aftermarket. With the premium brands Kolbenschmidt, Pierburg, TRW Engine Components and the BF brand, Motorservice offers its customers a wide and compre- hensive range of top quality products from a single source. As a problem solver for trade and repair shops, the corporation also offers an extensive service package. Motorservice customers benefit from the combined technical know-how of a large international auto- motive supplier. RHEINMETALL AUTOMOTIVE RENOWNED SUPPLIER TO THE INTERNATIONAL AUTOMOTIVE INDUSTRY Rheinmetall Automotive is the mobility division of the technology corporation Rheinmetall Group. With its premium brands Kolbenschmidt, Pierburg and Motorservice, Rheinmetall Automotive is a global leader in the relevant markets for air supply systems, emission control and pumps and in the development, manufacture and spare-parts supply of pistons, engine blocks and plain bearings. Low pollutant emissions, good fuel economy, reliability, quality and safety are the main driving forces behind the innova- tions of Rheinmetall Automotive. Edited by: Liability Motorservice, Technical Market Support All information in this brochure has been carefully researched and compiled. Nevertheless, it is possible that errors have occurred, information has been translated incorrectly, information is missing or the details Layout and production: provided have changed in the intervening time. As a result, we are unable to provide any guarantee nor to Motorservice, Marketing accept any legal liability for the accuracy, completeness, currency or quality of the information provided. -

Service Bulletin 13-079
Service Bulletin 13-079 May 18, 2016 51868-02139 Version 6 Warranty Extension: MIL Comes On with DTCs P0301 Thru P0304 Supersedes 13-079, dated September 12, 2015, to revise the information highlighted in yellow. AFFECTED VEHICLES Year Model Trim VIN Range 2010–12 Crosstour All V6 Check the iN VIN Status for Eligibility. REVISON SUMMARY • Under REPAIR PROCEDURE B – PISTON CLEANING AND RING REPLACEMENT, Replace Parts as Needed for Repair Procedure B, changed bolt count for the 12 x 163 Washer bolt (P/N 90005-PAA-A01) under Cyl 1-4 from 8 to 16. Also changed the bolt count for Connecting Rod Bolt from 8 to 6. CORRECTIVE ACTION 2010–11 Models: Complete the diagnosis procedure and if necessary, update the PCM software and replace the affected spark plugs. If the vehicle returns after the updated PCM software and spark plug replacement have been installed, clean the pistons and replace the pistons rings along with the spark plugs (Procedure B). NOTE: Make sure the software update has been completed, spark plugs replaced (Procedure A), and the vehicle returned to the customer before making any additional repairs. Failure to do so will result in claim review by your DPSM and possible debit. 2012 Models: Complete the diagnosis procedure and if necessary, clean the pistons and replace the pistons rings along with the spark plugs (Procedure B). BACKGROUND American Honda is announcing a powertrain warranty extension as a result of a settlement of a class action captioned, Soto et al.v. American Honda Motor Co., Inc., Case No. 3:12-cv-1377-SI (N.D. -

The Rotating Liner Engine (RLE) Diesel Prototype: Reducing Internal Engine Friction by About 40% Under Idle Conditions
applied sciences Article The Rotating Liner Engine (RLE) Diesel Prototype: Reducing Internal Engine Friction by about 40% under Idle Conditions Dimitrios Dardalis 1,*, Amiyo Basu 2,*, Matt J. Hall 2,* and Ronald D. Mattthews 2,* 1 RSET Inc., University of Texas at Austin, Austin, TX 78712, USA 2 University of Texas at Austin, Austin, TX 78712, USA * Correspondence: [email protected] (D.D.); [email protected] (A.B.); [email protected] (M.J.H.); [email protected] (R.D.M.) Abstract: The Rotating Liner Engine (RLE) concept is a design concept for internal combustion engines, where the cylinder liner rotates at a surface speed of 2–4 m/s in order to assist piston ring lubrication. Specifically, we have evidence from prior art and from our own research that the above rotation has the potential to eliminate the metal-to-metal contact/boundary friction that exists close to the piston reversal areas. This frictional source becomes a significant energy loss, especially in the compression/expansion part of the cycle, when the gas pressure that loads the piston rings and skirts is high. This paper describes the Diesel RLE prototype constructed from a Cummins 4BT and the preliminary observations from initial low load testing. The critical technical challenge, namely the rotating liner face seal, appears to be operating with negligible gas leakage and within the hydrodynamic lubrication regime for the loads tested (peak cylinder pressures of the order of 100 bar) and up to about 10 bar BMEP (brake mean effective pressure). Preliminary testing has proven that the metal-to-metal contact in the piston assembly mostly vanished, and a friction reduction at idle conditions of about 40% as extrapolated to a complete engine has taken place. -
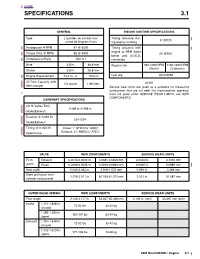
Specifications 3.1
3 HOME SPECIFICATIONS 3.1 GENERAL ENGINE IGNITION SPECIFICATIONS Type 2 cylinder, air cooled, four- Timing Advance dur- 5° BTDC stroke 45 Degree V-twin ing engine cranking Horsepower @RPM 91 @ 6200 Timing Advance with engine at RPM listed Torque ft-lbs @ RPM 85 @ 4900 20° BTDC below and V.O.E.S. Compression Ratio 10.0 to 1 connected Bore 3.5 in. 88.8 mm Regular idle 950-1050 RPM 1150-1250 RPM (World) (California) Stroke 3.8 in. 96.8 mm Engine Displacement 73.4 cu. in. 1203 cc Fast idle 2000 RPM Oil Tank Capacity (with 2.0 quarts 1.89 liters NOTE filter change) Service wear limits are given as a guideline for measuring components that are not new. For measurement specifica- tions not given under SERVICE WEAR LIMITS, see NEW COMPONENTS. CAMSHAFT SPECIFICATIONS Lift @ Valve (TDC) 0.458 in./0.458 in. Intake/Exhaust Duration @ 0.053 lift 224°/224° Intake/Exhaust Timing @ 0.053 lift Intake: 1° BTDC/43° ABDC Open/Close Exhaust: 41° BBDC/3° ATDC VALVE NEW COMPONENTS SERVICE WEAR LIMITS Fit in Exhaust 0.0015-0.0033 in. 0.0381-0.0838 mm 0.0040 in. 0.1016 mm guide Intake 0.0008-0.0026 in. 0.0203-0.0660 mm 0.0035 in. 0.0889 mm Seat width 0.040-0.062 in. 1.016-1.575 mm 0.090 in. 2.286 mm Stem protrusion from 1.975-2.011 in. 50.165-51.079 mm 2.031 in. 51.587 mm cylinder valve pocket OUTER VALVE SPRING NEW COMPONENTS SERVICE WEAR LIMITS Free length 2.105-2.177 in.