Table Olives: an Overview on Effects of Processing on Nutritional and Sensory Quality
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Change in Quality During Ripening of Olive Fruits and Related Oils Extracted from Three Minor Autochthonous Sardinian Cultivars
Emirates Journal of Food and Agriculture. 2019. 31(3): 196-205 doi: 10.9755/ejfa.2019.v31.i3.1923 http://www.ejfa.me/ RESEARCH ARTICLE Change in quality during ripening of olive fruits and related oils extracted from three minor autochthonous Sardinian cultivars Paola Conte1, Giacomo Squeo2, Graziana Difonzo2, Francesco Caponio2, Costantino Fadda1, Alessandra Del Caro1, Pietro Paolo Urgeghe1, Luigi Montanari1, Antonio Montinaro3, Antonio Piga1* 1Dipartimento di Agraria, Università Degli Studi di Sassari, Viale Italia 39/A, 07100 Sassari, Italy, 2Dipartimento di Scienze del Suolo, Della Pianta e Degli Alimenti, Sezione di Scienze e Tecnologie Alimentari, Università Degli Studi di Bari Aldo Moro, Via Amendola 165/A, 70126 Bari, Italy, 3LAORE Sardegna, Servizio Sviluppo delle filiere vegetali, Unità organizzativa tematica territoriale Produzioni vegetali ATO 2, Via Baldedda 11, 07100 Sassari, Italy ABSTRACT Ripening stage is one of the key factors in determining quality of olive fruits and related oils. This research, thus, was aimed to study the influence of three different harvesting times on the quality parameters of olives and related oils of three autochthonous Sardinian cultivars, Sivigliana da olio, Semidana, and Corsicana da olio. We evaluated several parameters in olive fruits (dry matter, oil content, total soluble solids, total polyphenol and antioxidant activity) and oils (legal indices, total chlorophylls and tocopherols, single polyphenols and volatile compounds, antioxidant activity). The results obtained in olive fruits showed that all the parameters changed significantly during ripening and seem to confirm that the best harvesting time is that selected by the growers, that is when 70% of olives has just turned dark-colored and the rest is green. -

Ph.D. Thesis Morrone
UNIVERSITA’ DEGLI STUDI DI PARMA Department of Food Science Ph. D. in Food Science and Technology Cycle XXVII Relationship between environmental features and extra virgin olive oil in north Sardinia Ph. D. Coordinator: Chiar.mo Prof. Furio Brighenti Tutor: Chiar.mo Prof. Andrea Fabbri Co-Tutors: Dott.ssa Annalisa Rotondi Dott. Tommaso Ganino Ph.D. Student: Lucia Morrone Lucia Morrone, 2015 Relationship between environmental features and extra virgin olive oil in north Sardinia PhD Thesis in Food Science and Technology. XXVII Cycle, University of Parma, ITALY. Thesis Supervisors: Prof. Andrea Fabbri – Department of Food Science, University of Parma Dr. Annalisa Rotondi – Institute of BIoMETeorology of the National Research Council (IBIMET – CNR), Bologna Dr. Tommaso Ganino - Department of Food Science, University of Parma PhD Coordinator : Prof. Furio Brighenti – Department of Food Science, University of Parma II To Maurizio III IV Preface and Acknowledgements The agri-food sector is a strategic asset for Italy, representing the 8,7% of GDP. The significance of this sector is not merely economic, even if the agri-food sector is an important item of GDP and it has always a positive mark in export. As a matter of fact, the agri-food sector has both a social and an environmental impact. In this regard, the valorisation of Italian agri food productions, the so- called Made-in-Italy Agri-Food, assumes a crucial importance. The extra virgin olive oil is one of the products that most personify the image of the Made-in-Italy Agri-Food. Notwithstanding a lot of people think at the Italian virgin olive oil like a one and definite product, it is a product having hundreds of chemical and sensory shades. -

Diapositiva 1
CNR - Istituto di Bioscienze e Biorisorse, Perugia Luciana Baldoni Composizione varietale degli oli di oliva mediante analisi del DNA I Metodi di Controllo – Il Controllo dei Metodi Torino, 9-10 Novembre 2017 Gli oli extra vergine di oliva L’ampia gamma di oli extra vergine di oliva è conseguenza: del grandissimo numero di varietà ancora in coltivazione, dell’area geografica di produzione e delle condizioni pedo-climatiche locali delle tecnologie di produzione, estrazione e stoccaggio L’olio di oliva è il prodotto alimentare sottoposto al maggior numero di contraffazioni in Europa Le più diffuse contraffazioni degli oli extra vergine di oliva si basano sulla diversa reputazione, notorietà e prezzo di certi oli rispetto ad altri (es. Olio Italiano 100%, DOP e IGP) Il principale bersaglio di questo tipo di contraffazioni sono le varietà impiegate per costruire l’olio Le analisi chimiche e fisiche classiche non sono in grado di rilevare le miscelazioni improprie degli oli Il patrimonio varietale dell’olivo Italia 538 42% del patrimonio mondiale Spagna 183 14% Francia 88 7% Grecia 52 4% Turchia 45 3,5% Tunisia 44 3,5% Algeria 41 3,2% Portogallo 24 1,9% Altri paesi 260 20% TOTALE 1.275 Perchè la caratterizzazione molecolare è necessaria? Confusione sull’identità varietale dovuta a diverse ragioni Varietà locali Varietà cosmopolite Varietà migranti Sinonimia (Frantoio-Raggiola) Omonimia (Rosciola) Toponimia (Nostrale di Rigali, Moraiolo dei Monti Martani) Morfonimia (Pendolino, Limona) Cultivar, Cloni? Ecotipi locali, Popolazioni varietali? -

Effect of Processing on Phenolic Composition of Olive Oil Products and Olive Mill By-Products and Possibilities for Enhancement of Sustainable Processes
processes Review Effect of Processing on Phenolic Composition of Olive Oil Products and Olive Mill By-Products and Possibilities for Enhancement of Sustainable Processes Fereshteh Safarzadeh Markhali CEB—Centre of Biological Engineering, Campus of Gualtar, University of Minho, 4710-057 Braga, Portugal; [email protected] or [email protected] Abstract: The bio-functional properties of olive oil products and by-products rely greatly on the proportions and types of the endogenous phenolics that may favorably/unfavorably change during various processing conditions. The olive oil industrial activities typically produce (i) olive oils, the main/marketable products, and (ii) olive mill by-products. The mechanical processing of olive oil extraction is making progress in some areas. However, the challenges inherent in the existing system, taking into consideration, the susceptibilities of phenolics and their biosynthetic variations during processing, hamper efforts to ascertain an ideal approach. The proposed innovative means, such as inclusion of emerging technologies in extraction system, show potential for sustainable development of olive oil processing. Another crucial factor, together with the technological advancements of olive oil extraction, is the valorization of olive mill by-products that are presently underused while having great potential for extended/high-value applications. A sustainable re-utilization of these Citation: Safarzadeh Markhali, F. valuable by-products helps contribute to (i) food and nutrition security and (ii) economic and Effect of Processing on Phenolic Composition of Olive Oil Products environmental sustainability. This review discusses typical processing factors responsible for the and Olive Mill By-Products and fate of endogenous phenolics in olive oil products/by-products and provides an overview of the Possibilities for Enhancement of possibilities for the sustainable processing to (i) produce phenolic-rich olive oil and (ii) optimally Sustainable Processes. -

Participants Catalogue
Participants Catalogue Food & Wine 2018 Page 1 of 105 Spain Alberto de Miguel S.A. - Conservas Emperatriz 5 Producer company: Canned seafood, canned vegetables and ready meals Spain Almazara Ecologica de La Rioja. S.L. 7 Producer company: Organic Extra Virgin Olive Oil - Organic Farming and D.O.P. Aceite de La Rioja Spain ARLUY S.L.U. 9 Producer company: ARLUY SLU Spain Arriezu Vineyards 10 Producer company: Spanish Organic wines from D.O.C. Rioja and D.O.Rueda Spain BODEGA DEL MONGE-GARBATI, S.C. 11 Producer company: D.O.Ca. Rioja Wine Spain Bodega Nuestra Señora de Vico/ Conservas Virto 12 Producer company: Products offered: Rioja Wine -young, crianza, reserva, garnacha, maceration carbonique, organic- and canned vegetables -artichokes, peas, pears, mixed pickles, vegetable marmelades, creams, organ- ics...- Spain BODEGA SEÑORIO DE LAS VIÑAS S.L, 14 Producer company: D.O.RIOJA Spain Bodegas Altanza, S.A. 15 Producer company: Bodegas Altanza, S.A. Spain BODEGAS ALVIA 17 Producer company: BODEGAS ALVIA Spain BODEGAS AMEZOLA DE LA MORA 19 Producer company: CHATEAU-LIKE BODEGA IN THE HEART OF THE RIOJA ALTA Spain Bodegas Castillo de Mendoza 20 Producer company: Noralba Blanco Organic Fermentado en Barrica 2017, Vitarán Tinto crianza 2015, Noralba Tinto crianza organic 2015, Castillo de Mendoza Reserva 2010, Castillo de Mendoza Autor organic 2015 and Evento de Castillo de Mendoza Autor 2004 (91+ Parker Points) Spain BODEGAS CASTILLO DE SAJAZARRA 22 Producer company: Crianza and Reserva Red Wines / White and Rosé Young Wines Spain Bodegas Covila 23 Producer company: Rioja Wine : Young Wines (White / Rose / Red), Crianza , Reserva, Gran Reserva & Author Wine Spain BODEGAS CUNA DE REYES, S.L. -

Terraolivo Regulation Organization
TERRAOLIVO REGULATION ORGANIZATION As part of putting together Olive oil, Nutrition and Health there will be a competition held in Israel, during the month of July known as Mediterranean International Olive Oil Competition - TerraOlivo PURPOSE Mediterranean International Olive Oil Competition is an International Competition of Extra Virgin Olive Oils Terraolivo, organized to reach the following objectives: ● Award the best Olive Oils Extra Virgin from all over the world. ● Promote all the nutritional benefits of Olive Oils EV directly to its consumers. ● Encourage the International market to notice the exceptional qualities of Olive Oils EV produced by different countries. ● Promote and make perceptible Olive Oil markets in the Mediterranean and the rest of the world. ● Spread all the advantages of having a Mediterranean diet. ● Introduce all winners to potential importers, in international markets, and to the media. ● Increase the international consumption of Olive Oils. STAGES The competition will have the following stages: ● Mediterranean International Olive Oil Competition: All Olive Oils will be tasted by a panel of professionals who will assess and classify them according to the COI. ● Guided Sampling: There will be a guided sampling of Olive Oils commented by the main specialists of the jury. ● First opening for winners to the press: All award winners will be presented to the International media and will be introduced to importers and distributors from worldwide markets. WHO CAN PARTICIPATE- ADMITTED PRODUCTS VERY IMPORTANT: The oils submitted should have a chemical analysis for free fatty acids completed no more than 120 days prior to submission. To be considered extra virgin olive oils, the free fatty acid level must not be more than 0.8% with a peroxide index of less than 20. -

Extra Virgin Olive Oils
Extra Virgin Olive Oils: French A L’Olivier Founded by a chemist in 1822, A L’Olivier is France’s most esteemed producer of specialty oils and vinegars. The picturesque Parisian shop still sells beautifully presented products in glass bottles, rustic stoneware crocks, and colorful tins. Olive Oil- A L’Olivier extra virgin olive oils are fragrant and fruity, with sweet, mild olive flavor. A L'Olivier Black and Fruity Extra Virgin Olive Oil Tin 250 ml 6 / case A L'Olivier Extra Virgin Olive Oil 250 ml 6 / case 500 ml 6 / case 1 L 6 / case A L'Olivier Extra Virgin Olive Oil Mini Drum 750 ml 6 / case A L’Olivier Extra Virgin Olive Oil Spray 200 ml 6 / case A L'Olivier Extra Virgin Olive Oil White Stoneware Crock 500 ml 6 / case Arnaud Arnaud Extra Virgin Olive Oil Salonenque, Blanquette, Verdale, Picholine, and Grossane olives 500 ml 12 / case Castelines From their orchards near Les Beaux, Jean Benoit and Catherine Hugues hand-pick a variety of olives just before they begin to blacken. These young olives are used to produce an olive oil that is light and delicate, with aromas of fresh grass and flavors of artichokes and almonds. Castelines also produces Late Harvest Fruite Noir olive oil, using only perfectly ripened native olives. The black olives are stored and lightly fermented to create a pleasantly sweet oil, with hints of truffle. Castelines Extra Virgin Olive Oil Salonenque, Aglandau, Grossane and Verdale olives 500 ml 6 / case 750 ml 6 / case Castelines Late Harvest Olive Oil (Fruite Noir) Salonenque, Aglandau, Grossane and Verdale olives 500 ml 6 / case Le Château d’Estoublon Le Château d’Estoublon Single Varietal Extra Virgin Olive Oil: Picholine 100% Picholine olives 500 ml 6 / case Le Château d’Estoublon Extra Virgin Olive Oil AOP: Vallée des Baux de Provence Béruguette, Bouteillan, Grossane, Salonenque and Picholine olives 500 ml 6 / case . -
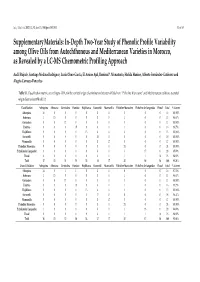
In-Depth Two-Year Study of Phenolic Profile Variability Among
Int. J. Mol. Sci. 2017, 18, 52; doi:10.3390/ijms18010052 S1 of S3 Supplementary Materials: In-Depth Two-Year Study of Phenolic Profile Variability among Olive Oils from Autochthonous and Mediterranean Varieties in Morocco, as Revealed by a LC-MS Chemometric Profiling Approach Aadil Bajoub, Santiago Medina-Rodríguez, Lucía Olmo-García, El Amine Ajal, Romina P. Monasterio, Hafida Hanine, Alberto Fernández-Gutiérrez and Alegría Carrasco-Pancorbo Table S1. Classification matrix, according to LDA, for the varietal origin discrimination between VOOs from “Picholine Marocaine” and Mediterranean cultivars (varietal origin discriminant Model 1). Classification Arbequina Arbosana Cornicabra Frantoio Hojiblanca Koroneiki Manzanilla Picholine Marocaine Picholine de Languedoc Picual Total % Correct Arbequina 16 0 0 0 0 0 0 0 0 0 16 100.00% Arbosana 1 13 0 0 0 0 0 1 0 0 15 86.67% Cornicabra 0 0 11 0 0 0 0 0 0 0 11 100.00% Frantoio 0 0 0 15 0 0 0 1 0 0 16 93.75% Hojiblanca 0 0 0 0 13 0 0 0 0 0 13 100.00% Koroneiki 0 0 0 0 0 18 0 0 0 0 18 100.00% Manzanilla 0 0 0 0 0 0 17 0 0 0 17 100.00% Picholine Marocaine 0 0 0 0 0 0 0 24 0 0 24 100.00% Picholine de Languedoc 0 0 0 0 0 0 0 3 17 0 20 85.00% Picual 0 0 0 0 0 0 0 1 1 16 18 88.89% Total 17 13 11 15 13 18 17 30 18 16 168 95.24% Cross-Validation Arbequina Arbosana Cornicabra Frantoio Hojiblanca Koroneiki Manzanilla Picholine Marocaine Picholine de Languedoc Picual Total % Correct Arbequina 14 0 1 1 0 0 0 0 0 0 16 87.50% Arbosana 1 13 0 0 0 0 0 1 0 0 15 86.67% Cornicabra 0 0 11 0 0 0 0 0 0 0 11 100.00% Frantoio 0 0 0 15 0 0 0 1 0 0 16 93.75% Hojiblanca 0 0 0 0 13 0 0 0 0 0 13 100.00% Koroneiki 0 0 0 0 1 17 0 0 0 0 18 94.44% Manzanilla 0 0 0 0 0 0 17 0 0 0 17 100.00% Picholine Marocaine 0 0 0 0 0 0 0 24 0 0 24 100.00% Picholine de Languedoc 1 0 0 0 0 0 0 3 16 0 20 80.00% Picual 0 0 0 0 0 0 0 1 1 16 18 88.89% Total 16 13 12 16 14 17 17 30 17 16 168 92.86% Int. -

Olive Oil Production in the Var Region of France, May, 1995
Olive Oil Production in the Var Region of France, May, 1995 In the south of France there are approximately 60 oil mills and cooperatives processing and retailing local olive oil today. They have a long history and tell the story of when olives were the dominant agricultural crop of the area. The Var region is at the center of what was once a thriving olive oil empire producing thousands of tons of olive oil each year. A devastating freeze in 1956 killed all of the olive trees down to the ground and most farmers replanted with the more profitable wine grapes for which France is so well known. Most of the mills in the region today still use the low technology stone mills and decantation processes their ancestors used. Unfortunately they have trouble finding enough olives nearby to sell much more than just to local residents, who bring their own olives for pressing. Mills that have modern equipment supplement their investment by bringing in olives from Spain and selling olive crafts, soaps, and canned table fruit in stores and restaurants adorned with antique processing equipment. Statistically, France is not a major producer of olive oil, processing an estimated 2,500 to 3,500 tons of olives in 1994. The Var region Chamber of Commerce economic development bulletin lists 11,136 farms in the region with an average size of just under 20 acres each. Vineyards represent 43% of the land and 47% of the earnings, cut flower production occurs on 1% of the land and represents 36% of the earnings. -

Future of Texas Olives AOOPA
THE FUTURE OF OLIVES IN TEXAS • Monte Nesbitt OLIVES ARE SOIL, DROUGHT AND SALT TOLERANT AND WE HAVE AN ABUNDANCE OF THOSE CHALLENGES IN TEXAS IS THERE AN OLIVE HISTORY IN TEXAS? Illustration by Silia Goetz, wsj.com KNOWN OLIVE HISTORY-TEXAS • Catholic archives in San Antonio indicate Spanish missionaries did not plant olives in Texas as they did in California (Denney, 1982). • Onderdonk (1900), important Texas nurseryman and fruit explorer wrote favorably of olives in San Luis Potosi, Mexico, but was otherwise silent about them. • Old trees dated 1920’s are reported to exist and bear fruit at places like Asherton & Galveston. • Mortensen (1938) described olive varieties introduced from California as “fair” for Wintergarden area and better for dooryard than commercial purposes. • Hartmann (1951) “Twenty-year effort in South Texas has failed to see fruiting” (Weslaco/Brownsville). Ernest Mortensen, Winterhaven, TX Exp. Station OLIVES ON TEXAS A&M CAMPUS 100’s of Manzanilla trees planted in 1974. Produced fruit in 1977, but were damaged by freezes in ‘78, ‘80, ‘81 (Denney, 1982) Thermal constraints on the productivity of olive (Olea europea L.) in the climates of olive-producing regions and of Texas, Thesis, Texas A&M Dept. of Horticultural Sciences, 1982 Dr. James Denney- deceased PUBLICATIONS • Denney, J.O. and G.R. McEachern. 1983. An analysis of several climatic temperature variables dealing with olive reproduction. J. Amer. Soc. Hort. Sci. 108:578-581. • Denney, J.O., McEachern, G.R. and J.F. Griffiths. 1985. Modeling the thermal adaptability of the olive (Olea europaea L.) in Texas. Agric. For. Meteorol. -

Roland Verhé
MinorMinor ComponentsComponents asas markersmarkers ofof oliveolive oiloil authenticityauthenticity andand qualityquality V. Van Hoed, R. Verhé, M. Andjelkovic Ghent University - Faculty of Bioscience Engineering - Department of Organic Chemistry [email protected] OverviewOverview I. Importance of analysis and authentification of olive oil II. Methods for determination of 1. Quality 2. Genuineness III. Authentication issues IV. Olive oil components used in this study V. Examples 1. Harvest time 2. Cultivar and geographic origin VI. Conclusions Importance of Analysis and Authentication of olive oils EVOO : mechanical extraction : unique composition and delicate aroma Lampante OO : needs refining Consumers preference: high quality + unchanged aroma and natural elements Higher price than other vegetable oils Danger of adulteration with cheaper ingredients = economical fraud = risk for consumers’ health Importance of Analysis and Authentication of olive oils Detect adulteration with cheaper ingredients Need for analysis and authentication methods Codex Alimentarius CA Commission Draft, 2003 European Commission (EC) EC Reg No 2568/1991 and amendment 1989/2003 International Olive Oil Council (IOOC) IOOC Trade Standards, 2003 = Official methods BUT sophisticated refining/adulteration Need for continual research = new methods, not (yet) evaluated by standardizing bodies, but proposed by researchers Methods for olive oil quality verification a) Methods included in the international Standards • Olive oil sampling and laboratory sample -

Genetic and Environmental Features for Oil Composition in Olive Varieties
OCL 2014, 21(5) D504 c A.J. Bervillé, C.M. Breton, Published by EDP Sciences 2014 OCL DOI: 10.1051/ocl/2014007 Oilseeds & fats Crops and Lipids Available online at: www.ocl-journal.org Research Article –Dossier Open Access OLIVE OIL Huile d’olive Genetic and environmental features for oil composition in olive varieties André Jean Bervillé1 and Catherine Marie Breton2,, 1 INRA, UMR DIAPC, 2 place Viala, Bât 33, 34060 Montpellier Cedex 2, France ossier 2 INRA, UMR-AGAP, équipe Davem, campus Supagro, Bat 21, 34060 Montpellier Cedex 1, France D Received 21 January 2014 – Accepted 13 February 2014 Abstract – Consumption of olive oil helps both prevent and cure heart disease. Olive oils vary in their fatty acid profiles as well as those of other secondary metabolites (phenols, sterols, and terpene compounds). We seek to distinguish the genetic bases from the environmental factors that cause these variations. The genetic base is indeed wide: varieties originate in different domestication occurrences, from different oleaster trees and in differing climatic regimes. With the aid of diagrams, we set out briefly the oil synthesis pathway for fruits in comparison with that of seeds, and the specific aspects of olive oil in particular. Varieties of olive have appeared that are adapted to regions with harsh conditions where the oleaster could not thrive. Environmental stresses have consequences on drupes and their oil profiles; these have been highlighted in European countries through the use of appellations. Whilst stresses tend to enhance the quality of the end product, they do however decrease final yields with potential negative impacts on olive growers’ incomes.