Iv the Thesis Work Is Dedicated to My Beloved Parents Whose Support and Prayers Make Possible This Achievement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1. Define: Unit Operation. Useful Physical Changes Occur in the Chemical Industry Is Known As a Unit Operation
INTREVIEW QUESTIONS 1. Define: Unit operation. Useful Physical changes occur in the chemical industry is known as a Unit Operation. Example: Distillation, Filtration, Drying, Extraction, Gas absorption, Crystallization, etc. 2. Define: Unit process. Useful Chemical changes with or without physical change occur in chemical industry are known as a Unit Process. Example: - Oxidation, Reduction, Alkylation, Sulfonation, Chlorination, etc. 3. Define: Boiling Point and Bubble Point. Boiling Point: -It is a temperature of a liquid at which the vapour pressure of the liquid is equal to atmospheric pressure. Bubble Point: -It is a temperature at which first bubble of vapour is formed. 4. When does liquid boil? When the vapour pressure of the liquid is equal to atmospheric pressure at that time liquid is boil. 5. Define: - Volatile Liquid. It is a tendency of a liquid to vaporize. 6. Acetone and water out of this which is more volatile and why? Acetone is more volatile than water because of boiling point of acetone (56.7 °C) is low compa re to boiling point of water (100° C) 7. What is the Relative Volatility? It is a ratio of concentration of more volatile component in vapour phase to liquid phase is called Relative Volatility 8. What is important of Relative Volatility? For separation of liquid mixture using distillation, Relative volatility should be more than 1. 9. Why is Reflux done in distillation column? and Define Reflux Ratio. For Increasing product purity. Reflux: -It is amount of distillate which is resend to distillation column is known as a reflux. Reflux Ratio: -Reflux ratio is the ratio of the portion of the overhead liquid product from a distillation column that is returned to the upper part of column to the portion of liquid collected as distillate. -

Multicomponent Distillation
01/12/2009 Multicomponent distillation F. Grisafi 1 01/12/2009 Introduction The problem of determining the stage and reflux requitirements for multicomponen t distill a tions ismuch more complex than for binary mixtures. With a multicomponent mixture, fixing one component composition does not uniquely determine theother component compositions and the stage temperature. Also when the feed contains more than two components it is not possible to specify the complete composition of the top and bottom products independently. The separation between the top and bottom products is usually specified by setting limits on two "key components", between which it is desired to make the separation. 2 01/12/2009 Calculation procedure The normal procedure for a typical problem is to solve the MESH (Material balance, Equilibrium, Summation and Heat) balance equations stage-by-stage, from the top and bottom of the column toward the feed point. For such a calculation to be exact, the compositions obtained from both the bottom-up and top-down calculations must mesh at the feed point and mesh the feed composition. The calculated compositions will depend on the compositions assumed for the top and bottom products at the commencement of the calculations. Though it ispossibletomatch the key components, theother components will not match unless the designer was particularly fortunate in choosing the trial top and bottom compositions. 3 01/12/2009 Calculation procedure For a comppyletely rigorous solution the compositions must be adjusted and the calculations repeated until a satisfactory match at the feed point is obtained by iterative trial-and-error calculations. Clearly, the greater the number of components, the more difficult the problem. -

Distillation 6
CHAPTER Energy Considerations in Distillation 6 Megan Jobson School of Chemical Engineering and Analytical Science, The University of Manchester, Manchester, UK CHAPTER OUTLINE 6.1 Introduction to energy efficiency ....................................................................... 226 6.1.1 Energy efficiency: technical issues................................................... 227 6.1.1.1 Heating.................................................................................... 227 6.1.1.2 Coolingdabove ambient temperatures...................................... 229 6.1.1.3 Coolingdbelow ambient temperatures ...................................... 230 6.1.1.4 Mechanical or electrical power.................................................. 232 6.1.1.5 Summarydtechnical aspects of energy efficiency ..................... 233 6.1.2 Energy efficiency: process economics............................................... 233 6.1.2.1 Heating.................................................................................... 233 6.1.2.2 Cooling..................................................................................... 235 6.1.2.3 Summarydprocess economics and energy efficiency ............... 237 6.1.3 Energy efficiency: sustainable industrial development........................ 237 6.2 Energy-efficient distillation ............................................................................... 237 6.2.1 Energy-efficient distillation: conceptual design of simple columns...... 238 6.2.1.1 Degrees of freedom in design .................................................. -

MATERIAL BALANCE NOTES Revision 3 Irven Rinard Department
MATERIAL BALANCE NOTES Revision 3 Irven Rinard Department of Chemical Engineering City College of CUNY and Project ECSEL October 1999 © 1999 Irven Rinard CONTENTS INTRODUCTION 1 A. Types of Material Balance Problems B. Historical Perspective I. CONSERVATION OF MASS 5 A. Control Volumes B. Holdup or Inventory C. Material Balance Basis D. Material Balances II. PROCESSES 13 A. The Concept of a Process B. Basic Processing Functions C. Unit Operations D. Modes of Process Operations III. PROCESS MATERIAL BALANCES 21 A. The Stream Summary B. Equipment Characterization IV. STEADY-STATE PROCESS MODELING 29 A. Linear Input-Output Models B. Rigorous Models V. STEADY-STATE MATERIAL BALANCE CALCULATIONS 33 A. Sequential Modular B. Simultaneous C. Design Specifications D. Optimization E. Ad Hoc Methods VI. RECYCLE STREAMS AND TEAR SETS 37 A. The Node Incidence Matrix B. Enumeration of Tear Sets VII. SOLUTION OF LINEAR MATERIAL BALANCE MODELS 45 A. Use of Linear Equation Solvers B. Reduction to the Tear Set Variables C. Design Specifications i VIII. SEQUENTIAL MODULAR SOLUTION OF NONLINEAR 53 MATERIAL BALANCE MODELS A. Convergence by Direct Iteration B. Convergence Acceleration C. The Method of Wegstein IX. MIXING AND BLENDING PROBLEMS 61 A. Mixing B. Blending X. PLANT DATA ANALYSIS AND RECONCILIATION 67 A. Plant Data B. Data Reconciliation XI. THE ELEMENTS OF DYNAMIC PROCESS MODELING 75 A. Conservation of Mass for Dynamic Systems B. Surge and Mixing Tanks C. Gas Holders XII. PROCESS SIMULATORS 87 A. Steady State B. Dynamic BILIOGRAPHY 89 APPENDICES A. Reaction Stoichiometry 91 B. Evaluation of Equipment Model Parameters 93 C. Complex Equipment Models 96 D. -

Review of Extractive Distillation. Process Design, Operation
Review of Extractive Distillation. Process design, operation optimization and control Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, Xinqiang You To cite this version: Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, et al.. Review of Extractive Distillation. Process design, operation optimization and control. Chemical Engineering Research and Design, Elsevier, 2019, 141, pp.229-271. 10.1016/j.cherd.2018.09.020. hal-02161920 HAL Id: hal-02161920 https://hal.archives-ouvertes.fr/hal-02161920 Submitted on 21 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive Toulouse Archive Ouverte OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/239894 Official URL: https://doi.org/10.1016/j.cherd.2018.09.020 To cite this version: Gerbaud, Vincent and Rodríguez-Donis, Ivonne and Hegely, Laszlo and Láng, Péter and Dénes, Ferenc and You, Xinqiang Review of Extractive Distillation. Process design, operation optimization and control. (2018) Chemical Engineering Research and Design, 141. -

Example: Multicomponent Distillation with Shortcut Methods Consider A
2 Example: multicomponent distillation with shortcut methods Consider a single distillation column as shown in Fig. 6-1. Short-cut methods have been developed that allow you to see the splits that can be achieved at different pressures, with different reflux ratios, and with a different number of stages. It is often useful to do a short-cut distillation calculation first, before doing the more rigorous plate- to-plate calculations, because the preliminary calculations give you an idea of what is reasonable. The methods are based on the relative volatility of the chemicals, and require you to identify two components that will be essentially separated (to the extent you specify). If you line up all the components in the order of their boiling points, and draw a line between two of them, the more volatile component is called the light key and the less volatile component is called the heavy key. B1 Figure 6-1. Distillation Column For this example, you choose the first column in the process shown in Fig. 5-4. In Aspen Plus you use the module DSTWU for the shortcut method; you also use RK-Soave as the physical property method, because it is a good one for hydrocarbons. The feed is 100 lb. moles/hr of propane, 300 lb. moles/hr of i-butane, and the other chemicals as listed in Table 5-1 or Table 6-6, at 138 psia and 75 ºF. The column operates at 138 psia with a reflux ratio of 10 (a wild1 guess initially, confirmed because the column worked). -

Distillation Theory
Chapter 2 Distillation Theory by Ivar J. Halvorsen and Sigurd Skogestad Norwegian University of Science and Technology Department of Chemical Engineering 7491 Trondheim, Norway This is a revised version of an article published in the Encyclopedia of Separation Science by Aca- demic Press Ltd. (2000). The article gives some of the basics of distillation theory and its purpose is to provide basic understanding and some tools for simple hand calculations of distillation col- umns. The methods presented here can be used to obtain simple estimates and to check more rigorous computations. NTNU Dr. ing. Thesis 2001:43 Ivar J. Halvorsen 28 2.1 Introduction Distillation is a very old separation technology for separating liquid mixtures that can be traced back to the chemists in Alexandria in the first century A.D. Today distillation is the most important industrial separation technology. It is particu- larly well suited for high purity separations since any degree of separation can be obtained with a fixed energy consumption by increasing the number of equilib- rium stages. To describe the degree of separation between two components in a column or in a column section, we introduce the separation factor: ()⁄ xL xH S = ------------------------T (2.1) ()x ⁄ x L H B where x denotes mole fraction of a component, subscript L denotes light compo- nent, H heavy component, T denotes the top of the section, and B the bottom. It is relatively straightforward to derive models of distillation columns based on almost any degree of detail, and also to use such models to simulate the behaviour on a computer. -

Distillation Accessscience from McgrawHill Education
6/19/2017 Distillation AccessScience from McGrawHill Education (http://www.accessscience.com/) Distillation Article by: King, C. Judson University of California, Berkeley, California. Last updated: 2014 DOI: https://doi.org/10.1036/10978542.201100 (https://doi.org/10.1036/10978542.201100) Content Hide Simple distillations Fractional distillation Vaporliquid equilibria Distillation pressure Molecular distillation Extractive and azeotropic distillation Enhancing energy efficiency Computational methods Stage efficiency Links to Primary Literature Additional Readings A method for separating homogeneous mixtures based upon equilibration of liquid and vapor phases. Substances that differ in volatility appear in different proportions in vapor and liquid phases at equilibrium with one another. Thus, vaporizing part of a volatile liquid produces vapor and liquid products that differ in composition. This outcome constitutes a separation among the components in the original liquid. Through appropriate configurations of repeated vaporliquid contactings, the degree of separation among components differing in volatility can be increased manyfold. See also: Phase equilibrium (/content/phaseequilibrium/505500) Distillation is by far the most common method of separation in the petroleum, natural gas, and petrochemical industries. Its many applications in other industries include air fractionation, solvent recovery and recycling, separation of light isotopes such as hydrogen and deuterium, and production of alcoholic beverages, flavors, fatty acids, and food oils. Simple distillations The two most elementary forms of distillation are a continuous equilibrium distillation and a simple batch distillation (Fig. 1). http://www.accessscience.com/content/distillation/201100 1/10 6/19/2017 Distillation AccessScience from McGrawHill Education Fig. 1 Simple distillations. (a) Continuous equilibrium distillation. -

Computer Simulation Applied to Studying Continuous Spirit Distillation and Product Quality Control
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Food Control 22 (2011) 1592e1603 Contents lists available at ScienceDirect Food Control journal homepage: www.elsevier.com/locate/foodcont Computer simulation applied to studying continuous spirit distillation and product quality control Fabio R.M. Batista, Antonio J.A. Meirelles* Laboratory EXTRAE, Department of Food Engineering, Faculty of Food Engineering, University of Campinas e UNICAMP, Campinas, Brazil article info abstract Article history: This work aims to study continuous spirit distillation by computational simulation, presenting some Received 7 June 2010 strategies of process control to regulate the volatile content. The commercial simulator Aspen (Plus and Received in revised form dynamics) was selected. A standard solution containing ethanol, water and 10 minor components rep- 2 March 2011 resented the wine to be distilled. A careful investigation of the vaporeliquid equilibrium was performed Accepted 8 March 2011 for the simulation of two different industrial plants. The simulation procedure was validated against experimental results collected from an industrial plant for bioethanol distillation. The simulations were Keywords: conducted with and without the presence of a degassing system, in order to evaluate the efficiency of Spirits fi Distillation this system in the control of the volatile content. To improve the ef ciency of the degassing system, fl Simulation a control loop based on a feedback controller was developed. The results showed that re ux ratio and Aspen Plus product flow rate have an important influence on the spirit composition. High reflux ratios and spirit Degassing flow rates allow for better control of spirit contamination. -

Unit Operations for Bioprocess Engineers
Unit Operations for Bioprocess Engineers Chenming (Mike) Zhang Department of Biological Systems Engineering Virginia Polytechnic Institute and State University Blacksburg, VA 24061 Abstract Unit Operations in Biological Systems Engineering was introduced into the curriculum at Virginia Tech in 2000. It is a lecture and laboratory combined course. The lectures and experiments covered in the course had a narrow focus before the author took over in 2002. To broaden the education for students selecting the Bioprocess Engineering option within the curriculum, the author has revised the content of the course to give the students an opportunity to understand that different unit operations can be applied in various industries, namely food, biochemical, and biotechnology. The experiments have been decreased from 14 to 8 so the students could have a better grasp of the theories and applications. Students’ responses showed that it is important to design the experiments in a way to stimulate their desire to learn and perform. The author found that it is possible to give the students a better view of the various unit operations in bioprocess engineering. Page 9.1342.1 Page 1 Introduction As defined by Shuler and Kargi (2002), “Bioprocess engineers are engineers working to apply the principles of various disciplines, such as chemical, mechanical, electrical, and industrial, to processes based on using living cells or subcomponents of such cells.” In other words, bioprocess engineers are engineers who process biological materials to produce useful goods for society. Without question, bioprocess engineering is a broad-based engineering discipline. As educators, it is our job to broaden the view of the students, so they can take advantage of numerous, diverse job opportunities presented to them when they finish their BS degree. -
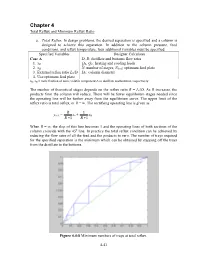
Minimum Reflux Ratio
Chapter 4 Total Reflux and Minimum Reflux Ratio a. Total Reflux . In design problems, the desired separation is specified and a column is designed to achieve this separation. In addition to the column pressure, feed conditions, and reflux temperature, four additional variables must be specified. Specified Variables Designer Calculates Case A D, B: distillate and bottoms flow rates 1. xD QR, QC: heating and cooling loads 2. xB N: number of stages, Nfeed : optimum feed plate 3. External reflux ratio L0/D DC: column diameter 4. Use optimum feed plate xD, xB = mole fraction of more volatile component A in distillate and bottoms, respectively The number of theoretical stages depends on the reflux ratio R = L0/D. As R increases, the products from the column will reduce. There will be fewer equilibrium stages needed since the operating line will be further away from the equilibrium curve. The upper limit of the reflux ratio is total reflux, or R = ∞. The rectifying operating line is given as R 1 yn+1 = xn + xD R +1 R +1 When R = ∞, the slop of this line becomes 1 and the operating lines of both sections of the column coincide with the 45 o line. In practice the total reflux condition can be achieved by reducing the flow rates of all the feed and the products to zero. The number of trays required for the specified separation is the minimum which can be obtained by stepping off the trays from the distillate to the bottoms. Figure 4.4-8 Minimum numbers of trays at total reflux. -

Making Downstream Processing Continuous and Robust a Virtual Roundtable S
BIOPROCESS TECHNICAL Making Downstream Processing Continuous and Robust A Virtual Roundtable S. Anne Montgomery, Cheryl Scott, and Peter Satzer, with Margit Holzer, Miriam Monge, Ralph Daumke, and Alexander Faude urrent biomanufacturing is driven to pursue continuous processing for cost reduction and increased productivity, Cespecially for monoclonal antibody (MAb) production and manufacturing. Although many technologies are now available and have been implemented in biodevelopment, implementation for large-scale production is still in its infancy. In a lively roundtable discussion at the BPI West conference in Santa Clara, CA (11 March 2019), participants touched on a number of important issues still to be resolved and technologies that are still in need of implementation at large scale. Below, moderator Peter Satzer (senior scientist RENTSCHLER BIOPHARMA (WWW.RENTSCHLER-BIOPHARMA.COM) with the Austrian Center of Industrial Biotechnology, Vienna) summarizes key points raised in that session. Based on Requirements for integration without the need for hold his highlights, BPI asked a number of Continuous Downstream tanks between unit operations requires industry representatives to comment on further exploration. those points further, and their Applications Chromatography Operations: Some responses follow. by Peter Satzer unit operations such as flow-through chromatography and single-pass, Minimizing Buffer and Hold Tanks: One tangential-flow filtration (SPTFF) can be critical parameter is integration of unit integrated easily into a continuous Product Focus: Biologics operations on manufacturing shop downstream process scheme at any floors using minimum numbers of point because they offer constant inflow Process Focus: Downstream buffer tanks and hold tanks. The benefit and outflow of material. Such operations processing of continuous manufacturing can be can be called truly or fully continuous.