Synthesis and Applications of Phosphatidylinositols and Their Analogues I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Suramin Alters Phosphoinositide Synthesis and Inhibits Growth Factor Receptor Binding in HT-29 Cells'
(CANCER RESEARCH 50. 6490-6496. October 15. 1990] Suramin Alters Phosphoinositide Synthesis and Inhibits Growth Factor Receptor Binding in HT-29 Cells' Reinhard Kopp2 and Andreas Pfeiffer Departments of Surgery and Internal Medicine II, Klinikum (irosshadern. University of Munich, West Germany ABSTRACT levels and a stimulation of calcium/calmodulin kinases. Di acylglycerol activates protein kinase C, a family of Ca2+-sensi- Initiation of cell growth frequently involves activation of growth factor tive and phospholipid-dependent isoenzymes, known to phos- receptor-coupled u rosine kinases and stimulation of the phosphoinositide phorylate regulatory proteins and to elevate cytosolic pH levels second messenger system. The antitrypanosomal and antifiliarial drug suramin has been shown to exert antiproliferative activities by inhibition (5, 6). Activation of protein kinase C by phorbol esters and of growth factor receptor binding. We therefore investigated the effect of elevation of intracellular calcium levels by calcium ionophores suramin on epidermal growth factor receptor-binding characteristics and, have been shown to be mitogenically active cofactors during the additionally, searched for effects on basal or cholinergically stimulated initiation of DNA synthesis (7-10). phospholipid metabolism in HT-29 cells. HT-29 colon carcinoma cells have recently been shown to Suramin caused a dose-dependent and noncompetitive inhibition of produce EGF/transforming growth factor «and insulin-like '"I-epidermal growth factor binding (concentration producing 50% inhi growth factor 1-like activities (11), indicating a possible auto bition, 44.2 Mg/ml)but did not alter muscarinic receptor binding. Suramin did not affect the basal '-'I* incorporation into phosphoinositides at crine proliferative effect of these growth factors. -

(4,5) Bisphosphate-Phospholipase C Resynthesis Cycle: Pitps Bridge the ER-PM GAP
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Topological organisation of the phosphatidylinositol (4,5) bisphosphate-phospholipase C resynthesis cycle: PITPs bridge the ER-PM GAP Shamshad Cockcroft and Padinjat Raghu* Dept. of Neuroscience, Physiology and Pharmacology, Division of Biosciences, University College London, London WC1E 6JJ, UK; *National Centre for Biological Sciences, TIFR-GKVK Campus, Bellary Road, Bangalore 560065, India Address correspondence to: Shamshad Cockcroft, University College London UK; Phone: 0044-20-7679-6259; Email: [email protected] Abstract Phospholipase C (PLC) is a receptor-regulated enzyme that hydrolyses phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) at the plasma membrane (PM) triggering three biochemical consequences, the generation of soluble inositol 1,4,5-trisphosphate (IP3), membrane– associated diacylglycerol (DG) and the consumption of plasma membrane PI(4,5)P2. Each of these three signals triggers multiple molecular processes impacting key cellular properties. The activation of PLC also triggers a sequence of biochemical reactions, collectively referred to as the PI(4,5)P2 cycle that culminates in the resynthesis of this lipid. The biochemical intermediates of this cycle and the enzymes that mediate these reactions are topologically distributed across two membrane compartments, the PM and the endoplasmic reticulum (ER). At the plasma membrane, the DG formed during PLC activation is rapidly converted to phosphatidic acid (PA) that needs to be transported to the ER where the machinery for its conversion into PI is localised. Conversely, PI from the ER needs to be rapidly transferred to the plasma membrane where it can be phosphorylated by lipid kinases to regenerate PI(4,5)P2. -

Non-Canonical Regulation of Phosphatidylserine Metabolism by a Phosphatidylinositol Transfer Protein and a Phosphatidylinositol 4-OH Kinase
bioRxiv preprint doi: https://doi.org/10.1101/696336; this version posted July 8, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Non-Canonical Regulation of Phosphatidylserine Metabolism by a Phosphatidylinositol Transfer Protein and a Phosphatidylinositol 4-OH Kinase Yaxi Wang1,2, Peihua Yuan2, Ashutosh Tripathi2, Martin Rodriguez1, Max Lönnfors2, Michal Eisenberg-Bord3, Maya Schuldiner3, and Vytas A. Bankaitis1,2,4† 1Department of Biochemistry and Biophysics Texas A&M University College Station, Texas 77843-2128 USA 2Department of Molecular and Cellular Medicine Texas A&M Health Science Center College Station, Texas 77843-1114 USA 3Department of Molecular Genetics Weizmann Institute of Science, Rehovot 7610001, Israel 4Department of Chemistry Texas A&M University College Station, Texas 77840 USA Key Words: phosphoinositides/ PITPs/ lipid kinases/ lipi metabolism/ membrane contact site † -- Corresponding author TEL: 979-436-0757 Email: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/696336; this version posted July 8, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. ABSTRACT The phosphatidylserine (PtdSer) decarboxylase Psd2 is proposed to engage in an endoplasmic reticulum (ER)-Golgi/endosome membrane contact site (MCS) that facilitates phosphatidylserine decarboxylation to phosphatidylethanomaine (PtdEtn) in Saccharomyces cerevisiae. -
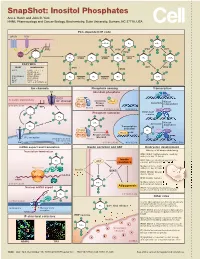
Snapshot: Inositol Phosphates Ace J
SnapShot: Inositol Phosphates Ace J. Hatch and John D. York HHMI, Pharmacology and Cancer Biology, Biochemistry, Duke University, Durham, NC 27710, USA PLC-dependent IP code GPCR RTK O O O O O O 5-PP-IP4 IP4 5-IP7 O O O O O O PIP2 O IP6K O IP6K O VIP1 O O O 2 O ITPK1 O 13 O PLC 2 O O O O O O O O 4 6 13 O 5 IP3 IPMK IP4 IPMK IP5 IPK1 IP6 1,5-IP8 4 6 O O 5 O O O O O O O O ENZYMES O O O O O O YEAST MAMMALIAN IP3K VIP1 IP6K IPMK PLC1 PLCβ, γ, δ, ε, ζ, η O - IP3KA, B, C - ITPK1 (IP56K) O O O O O O O O IPK2(ARG82) IPMK (IPK2) IP4 IP3 IP4 1-IP7 IPK1 IPK1 (IP5K) INPP5 ITPK1 KCS1 IP6K1, 2, 3 O O O O O O VIP1 VIP1, 2 (PPIP5K1, 2) O O Ion channels Phosphate sensing Transcription Cl- Abundant phosphate MCM1 ARG80 CIC3 P PLASMA MEMBRANE - Pho80 Cl channel Pho4 Kinase Kinase Assembly Pho85 independent CYTOPLASM activity 2 O PIP2 Pho81 13 CYTOPLASM NUCLEUS IPK2 ARG81 4 6 Phosphate starvation MCM1-ArgR O 5 O complex O O IP4 O O O O O O O 1-IP7 Kinase Activation dependent IP3 O O Transcription O O O activated Pho80 IP4 O X Pho4 O O Pho85 Kinase activity IP receptor blocked O 3 ENDOPLASMIC Pho81 RETICULUM Ca2+ CYTOPLASM NUCLEUS NUCLEUS mRNA export and translation Insulin secretion and AKT Embryonic development Translation termination Effects of IP kinase deficiency O IPMK (IPK2): Multiple defects, death by embryonic day 10 (mice) O O Insulin IPK1: Cillia are shortened and immotile IP6 AKT resistance causing patterning defects (zebrash) O O Multiple defects, death by Ribosome O embryonic day 8.5 (mice) GleI eRF1 Insulin GSK3β Dbp5 ITPK1 (IP56K): Neural tube -

(4,5)-Bisphosphate Destabilizes the Membrane of Giant Unilamellar Vesicles
5112 Biophysical Journal Volume 96 June 2009 5112–5121 Profilin Interaction with Phosphatidylinositol (4,5)-Bisphosphate Destabilizes the Membrane of Giant Unilamellar Vesicles Kannan Krishnan,† Oliver Holub,‡ Enrico Gratton,‡ Andrew H. A. Clayton,§ Stephen Cody,§ and Pierre D. J. Moens†* †Centre for Bioactive Discovery in Health and Ageing, School of Science and Technology, University of New England, Armidale, Australia; ‡Laboratory for Fluorescence Dynamics, Department of Biomedical Engineering, University of California, Irvine, California; and §Ludwig Institute for Cancer Research, Royal Melbourne Hospital, Victoria, Australia ABSTRACT Profilin, a small cytoskeletal protein, and phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P2] have been implicated in cellular events that alter the cell morphology, such as endocytosis, cell motility, and formation of the cleavage furrow during cytokinesis. Profilin has been shown to interact with PI(4,5)P2, but the role of this interaction is still poorly understood. Using giant unilamellar vesicles (GUVs) as a simple model of the cell membrane, we investigated the interaction between profilin and PI(4,5)P2. A number and brightness analysis demonstrated that in the absence of profilin, molar ratios of PI(4,5)P2 above 4% result in lipid demixing and cluster formations. Furthermore, adding profilin to GUVs made with 1% PI(4,5)P2 leads to the forma- tion of clusters of both profilin and PI(4,5)P2. However, due to the self-quenching of the dipyrrometheneboron difluoride-labeled PI(4,5)P2, we were unable to determine the size of these clusters. Finally, we show that the formation of these clusters results in the destabilization and deformation of the GUV membrane. -

Inositol Triphosphate-Triggered Calcium Release Blocks Lipid Exchange at Endoplasmic Reticulum- Golgi Contact Sites
ARTICLE https://doi.org/10.1038/s41467-021-22882-x OPEN Inositol triphosphate-triggered calcium release blocks lipid exchange at endoplasmic reticulum- Golgi contact sites Mouhannad Malek 1, Anna M. Wawrzyniak 1, Peter Koch1, Christian Lüchtenborg2, Manuel Hessenberger1, ✉ Timo Sachsenheimer2, Wonyul Jang 1, Britta Brügger 2 & Volker Haucke 1,3 fi 1234567890():,; Vesicular traf c and membrane contact sites between organelles enable the exchange of proteins, lipids, and metabolites. Recruitment of tethers to contact sites between the endo- plasmic reticulum (ER) and the plasma membrane is often triggered by calcium. Here we reveal a function for calcium in the repression of cholesterol export at membrane contact sites between the ER and the Golgi complex. We show that calcium efflux from ER stores induced by inositol-triphosphate [IP3] accumulation upon loss of the inositol 5-phosphatase INPP5A or receptor signaling triggers depletion of cholesterol and associated Gb3 from the cell surface, resulting in a blockade of clathrin-independent endocytosis (CIE) of Shiga toxin. This phenotype is caused by the calcium-induced dissociation of oxysterol binding protein (OSBP) from the Golgi complex and from VAP-containing membrane contact sites. Our findings reveal a crucial function for INPP5A-mediated IP3 hydrolysis in the control of lipid exchange at membrane contact sites. 1 Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP), Berlin, Germany. 2 Heidelberg University Biochemistry Center (BZH), Heidelberg ✉ University, Heidelberg, Germany. 3 Faculty of Biology, Chemistry and Pharmacy, Freie Universität Berlin, Berlin, Germany. email: [email protected] NATURE COMMUNICATIONS | (2021) 12:2673 | https://doi.org/10.1038/s41467-021-22882-x | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-22882-x ellular membrane homeostasis and the exchange of Results material between compartments can occur by vesicular INPP5A is required for Gb3-mediated Shiga toxin cell entry. -

Regulation of Signaling and Metabolism by Lipin-Mediated Phosphatidic Acid Phosphohydrolase Activity
biomolecules Review Regulation of Signaling and Metabolism by Lipin-mediated Phosphatidic Acid Phosphohydrolase Activity Andrew J. Lutkewitte and Brian N. Finck * Center for Human Nutrition, Division of Geriatrics and Nutritional Sciences, Department of Medicine, Washington University School of Medicine, Euclid Avenue, Campus Box 8031, St. Louis, MO 63110, USA; [email protected] * Correspondence: bfi[email protected]; Tel: +1-3143628963 Received: 4 September 2020; Accepted: 24 September 2020; Published: 29 September 2020 Abstract: Phosphatidic acid (PA) is a glycerophospholipid intermediate in the triglyceride synthesis pathway that has incredibly important structural functions as a component of cell membranes and dynamic effects on intracellular and intercellular signaling pathways. Although there are many pathways to synthesize and degrade PA, a family of PA phosphohydrolases (lipin family proteins) that generate diacylglycerol constitute the primary pathway for PA incorporation into triglycerides. Previously, it was believed that the pool of PA used to synthesize triglyceride was distinct, compartmentalized, and did not widely intersect with signaling pathways. However, we now know that modulating the activity of lipin 1 has profound effects on signaling in a variety of cell types. Indeed, in most tissues except adipose tissue, lipin-mediated PA phosphohydrolase activity is far from limiting for normal rates of triglyceride synthesis, but rather impacts critical signaling cascades that control cellular homeostasis. In this review, we will discuss how lipin-mediated control of PA concentrations regulates metabolism and signaling in mammalian organisms. Keywords: phosphatidic acid; diacylglycerol; lipin; signaling 1. Introduction Foundational work many decades ago by the laboratory of Dr. Eugene Kennedy defined the four sequential enzymatic steps by which three fatty acyl groups were esterified onto the glycerol-3-phosphate backbone to synthesize triglyceride [1]. -

Phosphatidylglycerol Incorporates Into Cardiolipin to Improve
www.nature.com/scientificreports OPEN Phosphatidylglycerol Incorporates into Cardiolipin to Improve Mitochondrial Activity and Inhibits Received: 3 July 2017 Accepted: 7 March 2018 Infammation Published: xx xx xxxx Wei-Wei Chen1, Yu-Jen Chao1, Wan-Hsin Chang1, Jui-Fen Chan1 & Yuan-Hao Howard Hsu1,2 Chronic infammation and concomitant oxidative stress can induce mitochondrial dysfunction due to cardiolipin (CL) abnormalities in the mitochondrial inner membrane. To examine the responses of mitochondria to infammation, macrophage-like RAW264.7 cells were activated by Kdo2-Lipid A (KLA) in our infammation model, and then the mitochondrial CL profle, mitochondrial activity, and the mRNA expression of CL metabolism-related genes were examined. The results demonstrated that KLA activation caused CL desaturation and the partial loss of mitochondrial activity. KLA activation also induced the gene upregulation of cyclooxygenase (COX)-2 and phospholipid scramblase 3, and the gene downregulation of COX-1, lipoxygenase 5, and Δ-6 desaturase. We further examined the phophatidylglycerol (PG) inhibition efects on infammation. PG supplementation resulted in a 358- fold inhibition of COX-2 mRNA expression. PG(18:1)2 and PG(18:2)2 were incorporated into CLs to considerably alter the CL profle. The decreased CL and increased monolysocardiolipin (MLCL) quantity resulted in a reduced CL/MLCL ratio. KLA-activated macrophages responded diferentially to PG(18:1)2 and PG(18:2)2 supplementation. Specifcally, PG(18:1)2 induced less changes in the CL/MLCL ratio than did PG(18:2)2, which resulted in a 50% reduction in the CL/MLCL ratio. However, both PG types rescued 20–30% of the mitochondrial activity that had been afected by KLA activation. -

Lipid–Protein and Protein–Protein Interactions in the Pulmonary Surfactant System and Their Role in Lung Homeostasis
International Journal of Molecular Sciences Review Lipid–Protein and Protein–Protein Interactions in the Pulmonary Surfactant System and Their Role in Lung Homeostasis Olga Cañadas 1,2,Bárbara Olmeda 1,2, Alejandro Alonso 1,2 and Jesús Pérez-Gil 1,2,* 1 Departament of Biochemistry and Molecular Biology, Faculty of Biology, Complutense University, 28040 Madrid, Spain; [email protected] (O.C.); [email protected] (B.O.); [email protected] (A.A.) 2 Research Institut “Hospital Doce de Octubre (imasdoce)”, 28040 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-913944994 Received: 9 May 2020; Accepted: 22 May 2020; Published: 25 May 2020 Abstract: Pulmonary surfactant is a lipid/protein complex synthesized by the alveolar epithelium and secreted into the airspaces, where it coats and protects the large respiratory air–liquid interface. Surfactant, assembled as a complex network of membranous structures, integrates elements in charge of reducing surface tension to a minimum along the breathing cycle, thus maintaining a large surface open to gas exchange and also protecting the lung and the body from the entrance of a myriad of potentially pathogenic entities. Different molecules in the surfactant establish a multivalent crosstalk with the epithelium, the immune system and the lung microbiota, constituting a crucial platform to sustain homeostasis, under health and disease. This review summarizes some of the most important molecules and interactions within lung surfactant and how multiple lipid–protein and protein–protein interactions contribute to the proper maintenance of an operative respiratory surface. Keywords: pulmonary surfactant film; surfactant metabolism; surface tension; respiratory air–liquid interface; inflammation; antimicrobial activity; apoptosis; efferocytosis; tissue repair 1. -

Studies of the Structure of Potassium Channel Kcsa in the Open Conformation and the Effect of Anionic Lipids on Channel Inactivation
Studies of the structure of potassium channel KcsA in the open conformation and the effect of anionic lipids on channel inactivation Dongyu (Allison) Zhang Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2019 © 2019 Dongyu Zhang All rights reserved Abstract Studies of the structure of potassium channel KcsA in the open conformation and the effect of anionic lipids on channel inactivation Dongyu Zhang Membrane proteins play a vital role in cellular processes. In this thesis, we use KcsA, a prokaryotic potassium channel, as a model to investigate the gating mechanism of ion channels and the effect of anionic lipids on the channel activity using solid-state NMR spectroscopy. KcsA activity is known to be highly dependent on the presence of negatively charged lipids. Multiple crystal structures combined with biochemistry assays suggest that KcsA is co-purified with anionic lipids with phosphatidylglycerol headgroup. Here, we identified this specifically bound, isotopically labeled lipid in the protein 13C-13C correlation spectra. Our results reveal that the lipid cross peaks show stronger intensity when the channel is in the inactivated state compared to the activated state, which indicates a stronger protein-lipid interaction when KcsA is inactivated. In addition, our data shows that including anionic lipids into proteoliposomes leads to a weaker potassium ion affinity at the selectivity filter. Considering ion loss as a model of inactivation, our results suggest anionic lipids promote channel inactivation. However, the surface charge is not the only physical parameter that regulates channel gating or conformational preference. -

The Role of Phosphatidylserine in Recognition of Apoptotic Cells by Phagocytes
Cell Death and Differentiation (1998) 5, 551 ± 562 1998 Stockton Press All rights reserved 13509047/98 $12.00 http://www.stockton-press.co.uk/cdd Review The role of phosphatidylserine in recognition of apoptotic cells by phagocytes Valerie A. Fadok1,2, Donna L. Bratton1, S. Courtney Frasch1, epithelial cells and vascular smooth muscle cells). To date, Mary L. Warner1 and Peter M. Henson1 there have been a number of receptors described for macrophages and other cells which bind to apoptotic cells 1 Department of Pediatrics, National Jewish Medical and Research Center, 1400 and mediate their uptake. These include lectin-like receptors Jackson Street, Denver, Colorado 80206 USA (Duvall et al, 1985, Dini et al, 1992; 1995; Hall et al, 1994; 2 corresponding author: tel: 1-303-398-1281 fax: 1-303-398-1381 Morris et al, 1994; Falasca et al, 1996), the vitronectin receptor email: [email protected] avb3 (Savill et al, 1990; Hall et al, 1994; Hughes et al, 1997), CD36 (Savill et al, 1992), an uncharacterized phosphatidyl- Received: 15.10.97; revised: 23.3.98; accepted: 2.4.98 Edited by M. Piacentini serine-recognizing receptor (Fadok et al, 1992a,b, 1993; Pradhan et al, 1997), CD14 (Flora and Gregory 1994; Devitt et al, 1998), and scavenger receptors (Sambrano and Steinberg, Abstract 1995; Fukasawa et al, 1996; Platt et al, 1996; Murao et al, 1997). The ABC1 transporter, also involved in uptake of Exposure of phosphatidylserine on the outer leaflet of the mammalian apoptotic cells, has recently been shown to plasma membrane is a surface change common to many mediate anion transport (Luciani and Chimini, 1996; Becq et apoptotic cells. -

GRAS Notice No. GRN 000728, FDA Has No Questions, Phospholipase C
.. .. Janet Oesterling Novozymes North America, Inc. 77 Perry Chapel Church Road Franklinton, NC 27525 Re: GRAS Notice No. GRN 000728 Dear Ms. Oesterling: The Food and Drug Administration (FDA, we) completed our evaluation of GRN 000728. We received Novozymes North America, Inc.’s (Novozymes’) notice on August 25, 2017, and filed it on September 8, 2017. We received an amendment containing additional safety information on October 11, 2017. The subject of the notice is phosphoinositide phospholipase C enzyme preparation produced by Bacillus licheniformis expressing a modified synthetic gene encoding a variant of the wild-type phosphoinositide phospholipase C from Pseudomonas sp. 62186 (phosphoinositide phospholipase C enzyme preparation) for use as an enzyme in the degumming of vegetable oils at a maximum level of 1.4 mg Total Organic Solids (TOS)/kg oil. The notice informs us of Novozymes’ view that this use of phosphoinositide phospholipase C enzyme preparation is GRAS through scientific procedures. Commercial enzyme preparations that are used in food processing typically contain an enzyme component that catalyzes the chemical reaction as well as substances used as stabilizers, preservatives, or diluents. Enzyme preparations may also contain components derived from the production organism and from the manufacturing process, e.g., constituents of the fermentation media or the residues of processing aids. Novozymes’ notice provides information about the components in the phosphoinositide phospholipase C enzyme preparation. Per the classification system of enzymes established by the International Union of Biochemistry and Molecular Biology, phosphoinositide phospholipase C is identified by the Enzyme Commission Number 3.1.4.11. The accepted name is phosphoinositide phospholipase C.