AG-348 Enhances Pyruvate Kinase Activity in Red Blood Cells from Patients with Pyruvate Kinase Deficiency
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phenotypic and Molecular Genetic Analysis of Pyruvate Kinase Deficiency in a Tunisian Family
The Egyptian Journal of Medical Human Genetics (2016) 17, 265–270 HOSTED BY Ain Shams University The Egyptian Journal of Medical Human Genetics www.ejmhg.eg.net www.sciencedirect.com ORIGINAL ARTICLE Phenotypic and molecular genetic analysis of Pyruvate Kinase deficiency in a Tunisian family Jaouani Mouna a,1,*, Hamdi Nadia a,1, Chaouch Leila a, Kalai Miniar a, Mellouli Fethi b, Darragi Imen a, Boudriga Imen a, Chaouachi Dorra a, Bejaoui Mohamed b, Abbes Salem a a Laboratory of Molecular and Cellular Hematology, Pasteur Institute, Tunis, Tunisia b Service d’Immuno-He´matologie Pe´diatrique, Centre National de Greffe de Moelle Osseuse, Tunis, Tunisia Received 9 July 2015; accepted 6 September 2015 Available online 26 September 2015 KEYWORDS Abstract Pyruvate Kinase (PK) deficiency is the most frequent red cell enzymatic defect responsi- Pyruvate Kinase deficiency; ble for hereditary non-spherocytic hemolytic anemia. The disease has been studied in several ethnic Phenotypic and molecular groups. However, it is yet an unknown pathology in Tunisia. We report here, the phenotypic and investigation; molecular investigation of PK deficiency in a Tunisian family. Hemolytic anemia; This study was carried out on two Tunisian brothers and members of their family. Hematolog- Hydrops fetalis; ical, biochemical analysis and erythrocyte PK activity were performed. The molecular characteriza- PKLR mutation tion was carried out by gene sequencing technique. The first patient died few hours after birth by hydrops fetalis, the second one presented with neonatal jaundice and severe anemia necessitating urgent blood transfusion. This severe clinical pic- ture is the result of a homozygous mutation of PKLR gene at exon 8 (c.1079G>A; p.Cys360Tyr). -

Non-Commercial Use Only
only use Non-commercial 14th International Conference on Thalassaemia and Other Haemoglobinopathies 16th TIF Conference for Patients and Parents 17-19 November 2017 • Grand Hotel Palace, Thessaloniki, Greece only use For thalassemia patients with chronic transfusional iron overload... Make a lasting impression with EXJADENon-commercial film-coated tablets The efficacy of deferasirox in a convenient once-daily film-coated tablet Please see your local Novartis representative for Full Product Information Reference: EXJADE® film-coated tablets [EU Summary of Product Characteristics]. Novartis; August 2017. Important note: Before prescribing, consult full prescribing information. iron after having achieved a satisfactory body iron level and therefore retreatment cannot be recommended. ♦ Maximum daily dose is 14 mg/kg body weight. ♦ In pediatric patients the Presentation: Dispersible tablets containing 125 mg, 250 mg or 500 mg of deferasirox. dosing should not exceed 7 mg/kg; closer monitoring of LIC and serum ferritin is essential Film-coated tablets containing 90 mg, 180 mg or 360 mg of deferasirox. to avoid overchelation; in addition to monthly serum ferritin assessments, LIC should be Indications: For the treatment of chronic iron overload due to frequent blood transfusions monitored every 3 months when serum ferritin is ≤800 micrograms/l. (≥7 ml/kg/month of packed red blood cells) in patients with beta-thalassemia major aged Dosage: Special population ♦ In moderate hepatic impairment (Child-Pugh B) dose should 6 years and older. ♦ Also indicated for the treatment of chronic iron overload due to blood not exceed 50% of the normal dose. Should not be used in severe hepatic impairment transfusions when deferoxamine therapy is contraindicated or inadequate in the following (Child-Pugh C). -

Hb S/Beta-Thalassemia
rev bras hematol hemoter. 2 0 1 5;3 7(3):150–152 Revista Brasileira de Hematologia e Hemoterapia Brazilian Journal of Hematology and Hemotherapy www.rbhh.org Scientific Comment ଝ The compound state: Hb S/beta-thalassemia ∗ Maria Stella Figueiredo Universidade Federal de São Paulo (UNIFESP), São Paulo, SP, Brazil Sickle cell disease (SCD) results from a single amino acid small increase in Hb concentration and in packed cell volume. substitution in the gene encoding the -globin subunit However, these effects are not accompanied by any reduction  ( 6Glu > Val) that produces the abnormal hemoglobin (Hb) in vaso-occlusive events, probably due to the great number of named Hb S. SCD has different genotypes with substantial Hb S-containing red blood cells resulting in increased blood 1,2 4,5 variations in presentation and clinical course (Table 1). The viscosity. combination of the sickle cell mutation and beta-thalassemia A confusing diagnostic problem is the differentiation 0  (-Thal) mutation gives rise to a compound heterozygous con- of Hb S/ -Thal from sickle cell anemia associated with ␣ dition known as Hb S/ thalassemia (Hb S/-Thal), which was -thalassemia. Table 2 shows that hematologic and elec- 3 first described in 1944 by Silvestroni and Bianco. trophoretic studies are unable to distinguish between the two The polymerization of deoxygenated Hb S (sickling) is the conditions and so family studies and DNA analysis are needed 5 primary event in the molecular pathogenesis of SCD. How- to confirm the diagnosis. +  ever, this event is highly dependent on the intracellular Hb In Hb S/ -Thal, variable amounts of Hb A dilute Hb S and composition; in other words, it is dependent on the concen- consequently inhibit polymerization-induced cellular dam- tration of Hb S, and type and concentration of the other types age. -

Non-Transfusion-Dependent Thalassemias
REVIEW ARTICLES Non-transfusion-dependent thalassemias Khaled M. Musallam, 1 Stefano Rivella, 2 Elliott Vichinsky, 3 and Eliezer A. Rachmilewitz, 4 1Department of Medicine and Medical Specialties, IRCCS Ca’ Granda Foundation Maggiore Policlinico Hospital, University of Milan, Milan, Italy; 2Department of Pediatrics, Division of Hematology/Oncology, Weill Medical College of Cornell University, New York, NY, USA; 3Department of Hematology and Oncology, Children’s Hospital and Research Center Oakland, Oakland, CA, USA; and 4Department of Hematology, Wolfson Medical Center, Holon, Israel ABSTRACT Non-transfusion-dependent thalassemias include a variety of phenotypes that, unlike patients with beta ( β)-tha - lassemia major, do not require regular transfusion therapy for survival. The most commonly investigated forms are β-thalassemia intermedia, hemoglobin E/ β-thalassemia, and α-thalassemia intermedia (hemoglobin H disease). However, transfusion-independence in such patients is not without side effects. Ineffective erythropoiesis and peripheral hemolysis, the hallmarks of disease process, lead to a variety of subsequent pathophysiologies including iron overload and hypercoagulability that ultimately lead to a number of serious clinical morbidities. Thus, prompt and accurate diagnosis of non-transfusion-dependent thalassemia is essential to ensure early intervention. Although several management options are currently available, the need to develop more novel therapeutics is jus - tified by recent advances in our understanding of the mechanisms of disease. Such efforts require wide interna - tional collaboration, especially since non-transfusion-dependent thalassemias are no longer bound to low- and middle-income countries but have spread to large multiethnic cities in Europe and the Americas due to continued migration. Introduction survival, although they may require occasional or even fre - quent transfusions in certain clinical settings and usually for Inherited hemoglobin disorders can be divided into two defined periods of time (Figure 1). -

The Variable Manifestations of Disease in Pyruvate Kinase Deficiency and Their Management
REVIEW ARTICLE The variable manifestations of disease in pyruvate kinase deficiency and their Ferrata Storti Foundation management Hanny Al-Samkari,1 Eduard J. van Beers,2 Kevin H.M. Kuo,3 Wilma Barcellini,4 Paola Bianchi,4 Andreas Glenthøj,5 María del Mar Mañú Pereira,6 Richard van Wijk,7 Bertil Glader8 and Rachael F. Grace9 1Division of Hematology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; 2Van Creveldkliniek, University Medical Centre Utrecht, University of Utrecht, Utrecht, the Netherlands; 3Division of Hematology, University of Toronto, University Health Haematologica 2020 Network, Toronto, Ontario, Canada; 4UOS Ematologia, Fisiopatologia delle Anemie, Volume 105(9):2229-2239 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; 5Department of Hematology, Herlev and Gentofte Hospital, Herlev, Denmark; 6Translational Research in Rare Anaemia Disorders, Vall d'Hebron Institut de Recerca (VHIR), Barcelona, Spain; 7Department of Clinical Chemistry & Hematology, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands; 8Lucile Packard Children’s Hospital, Stanford University School of Medicine, Palo Alto, CA, USA and 9Dana/Farber Boston Children's Cancer and Blood Disorders Center, Harvard Medical School, Boston, MA, USA. ABSTRACT yruvate kinase deficiency (PKD) is the most common cause of chron- ic hereditary non-spherocytic hemolytic anemia and results in a Pbroad spectrum of disease. The diagnosis of PKD requires a high index of suspicion and judicious use of laboratory tests that may not always be informative, including pyruvate kinase enzyme assay and genetic analysis of the PKLR gene. A significant minority of patients with PKD have occult mutations in non-coding regions of PKLR which are missed on standard genetic tests. -

Iron Deficiency and the Anemia of Chronic Disease
Thomas G. DeLoughery, MD MACP FAWM Professor of Medicine, Pathology, and Pediatrics Oregon Health Sciences University Portland, Oregon [email protected] IRON DEFICIENCY AND THE ANEMIA OF CHRONIC DISEASE SIGNIFICANCE Lack of iron and the anemia of chronic disease are the most common causes of anemia in the world. The majority of pre-menopausal women will have some element of iron deficiency. The first clue to many GI cancers and other diseases is iron loss. Finally, iron deficiency is one of the most treatable medical disorders of the elderly. IRON METABOLISM It is crucial to understand normal iron metabolism to understand iron deficiency and the anemia of chronic disease. Iron in food is largely in ferric form (Fe+++ ) which is reduced by stomach acid to the ferrous form (Fe++). In the jejunum two receptors on the mucosal cells absorb iron. The one for heme-iron (heme iron receptor) is very avid for heme-bound iron (absorbs 30-40%). The other receptor - divalent metal transporter (DMT1) - takes up inorganic iron but is less efficient (1-10%). Iron is exported from the enterocyte via ferroportin and is then delivered to the transferrin receptor (TfR) and then to plasma transferrin. Transferrin is the main transport molecule for iron. Transferrin can deliver iron to the marrow for the use in RBC production or to the liver for storage in ferritin. Transferrin binds to the TfR on the cell and iron is delivered either for use in hemoglobin synthesis or storage. Iron that is contained in hemoglobin in senescent red cells is recycled by binding to ferritin in the macrophage and is transferred to transferrin for recycling. -
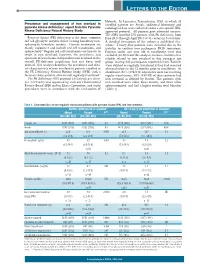
Prevalence and Management of Iron Overload in Pyruvate Kinase Deficiency
LETTERS TO THE EDITOR Helsinki. In Lancaster, Pennsylvania, USA, in which all Prevalence and management of iron overload in enrolled patients are Amish, additional laboratory and pyruvate kinase deficiency: report from the Pyruvate radiological data were collected under a site-specific IRB- Kinase Deficiency Natural History Study approved protocol. All patients gave informed consent. The NHS enrolled 278 patients with PK deficiency from Pyruvate kinase (PK) deficiency is the most common June 2014 through April 2017 at 31 centers in 6 countries. red cell glycolytic enzyme defect causing hereditary non- A detailed description of the cohort is published else- spherocytic hemolytic anemia. Current treatments are where.2 Twenty-four patients were excluded due to the mainly supportive and include red cell transfusions and inability to confirm two pathogenic PKLR mutations. splenectomy.1 Regular red cell transfusions are known to Patients under one year old at enrollment were also result in iron overload; however, the prevalence and excluded (n=12) from this analysis, because ferritin is less spectrum of transfusion-independent iron overload in the reliably related to iron overload in this youngest age overall PK-deficient population has not been well group, leaving 242 participants reported herein. Patients defined. This analysis describes the prevalence and clini- were defined as regularly transfused if they had received cal characteristics of iron overload in patients enrolled in ≥6 transfusions in the 12 months prior to enrollment. At the PK Deficiency Natural History Study (NHS) with a enrollment, 82% (198/242) of patients were not receiving focus on those patients who are not regularly transfused.2 regular transfusions; 38% (53/138) of these patients had The PK deficiency NHS protocol (clinicaltrials.gov identi- iron overload as defined by ferritin. -

The Coexistence of Polycythemia Vera and Iron Deficiency Anemia Somchai Insiripong1, Wattana Insiripong2
CASE REPORT The Coexistence of Polycythemia Vera and Iron Deficiency Anemia Somchai Insiripong1, Wattana Insiripong2 1Department of Medicine, Saint Mary Hospital, Nakhon Ratchasima 30000, Thailand, 2Department of General Practice, NopparatRajathanee Hospital, Khanna Yao, Bangkok 10230, Thailand ABSTRACT Polycythemia vera (PV) is a clonal myeloproliferative neoplasm mainly characterized by an abnormal increase of erythroid precursor cells leading to increased red blood cells (RBC) production that is opposite to iron deficiency anemia (IDA) of which the RBC production is decreased due to iron deficiency. This report was aimed to present one patient who had coexistence of these two opposite entities of the RBC production. She was a 47-year-old Thai who was admitted because of acute coronary syndrome and she was accidentally found to have microcytosis of RBC despite normal hemoglobin (Hb) concentration, Hb 14.7 g%, mean corpuscular volume (MCV) 70.0 fL, white blood cells 12,400/mm3, and platelet 401,000/mm3. The Hb analysis showed only A2A, with normal Hb A2 percentage. The polymerase chain reaction for alpha thalassemia-1 genotype was tested negative. Due to neither alpha- nor beta-thalassemia trait detected, the iron study was performed: Serum ferritin 6.1 ng/mL, serum iron 64 ug/dl, and total iron binding capacity 198 ug/dl. The iron storage was seemingly insufficient; hence, iron supplement was started and continued for 4 months. Her blood tests showed: Hb 18.3 g%, MCV 87.2 fl, serum ferritin 31.7 ng/ml, erythropoietin <1 IU/l, positive JAK2 V617F mutation, and normal oxygen saturation. The diagnosis of PV was definitely concluded and she was finally treated with hydroxyurea and occasional phlebotomy. -

Sickle Beta Plus Thalassemia Disease
Sickle Beta Plus Thalassemia Disease WHAT IS SICKLE BETA PLUS THALASSEMIA (Hb Sβ+ thal) Sickle Beta + Thalassemia (Sickle BA-ta Plus Thal-a-SEE-me-a) is a "mild" form of sickle cell disease. Your child's red blood cells contain an abnormal hemoglobin called hemoglobin S or sickle hemoglobin in addition to a small amount of the normal hemoglobin called hemoglobin A. The red blood cells have a defect called beta plus thalassemia, which results in cells which are small in size and more pale than usual. Instead of appearing round or donut shaped, your child's red blood cells are somewhat small, pale, and misshapen. Some may appear sickled or banana shaped. Because sickle beta plus thalassemia is inherited, it is a lifelong disorder. There is no treatment or cure. Your child will always have a mild anemia or slightly low blood count. This may cause occasional tiredness or weakness. HOW DID MY CHILD GET SICKLE BETA PLUS THALASSEMIA When one parent has Sickle Trait (AS) and the other parent has Beta Thalassemia Plus Trait (AT+), them is a l-in-4 chance (25 percent) the baby will have normal hemoglobin (AA), Sickle Trait (AS), Beta Thalassemia Plus Trait (AT+), or Sickle Beta Plus Thalassemia (ST +). These chances remain the same for each pregnancy. PROBLEMS SEEN IN CHILDREN WITH SICKLE BETA PLUS THALASSEMIA Painful episodes can occur with sickle-thalassemia. The sickled red blood cells in sickle-thalassemia, somewhat like those in sickle cell anemia, are rigid and stiff and may sometimes cause "log jams" in the small blood vessels in the bones, organs, and other parts of the body. -

Inborn Defects in the Antioxidant Systems of Human Red Blood Cells
Free Radical Biology and Medicine 67 (2014) 377–386 Contents lists available at ScienceDirect Free Radical Biology and Medicine journal homepage: www.elsevier.com/locate/freeradbiomed Review Article Inborn defects in the antioxidant systems of human red blood cells Rob van Zwieten a,n, Arthur J. Verhoeven b, Dirk Roos a a Laboratory of Red Blood Cell Diagnostics, Department of Blood Cell Research, Sanquin Blood Supply Organization, 1066 CX Amsterdam, The Netherlands b Department of Medical Biochemistry, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands article info abstract Article history: Red blood cells (RBCs) contain large amounts of iron and operate in highly oxygenated tissues. As a result, Received 16 January 2013 these cells encounter a continuous oxidative stress. Protective mechanisms against oxidation include Received in revised form prevention of formation of reactive oxygen species (ROS), scavenging of various forms of ROS, and repair 20 November 2013 of oxidized cellular contents. In general, a partial defect in any of these systems can harm RBCs and Accepted 22 November 2013 promote senescence, but is without chronic hemolytic complaints. In this review we summarize the Available online 6 December 2013 often rare inborn defects that interfere with the various protective mechanisms present in RBCs. NADPH Keywords: is the main source of reduction equivalents in RBCs, used by most of the protective systems. When Red blood cells NADPH becomes limiting, red cells are prone to being damaged. In many of the severe RBC enzyme Erythrocytes deficiencies, a lack of protective enzyme activity is frustrating erythropoiesis or is not restricted to RBCs. Hemolytic anemia Common hereditary RBC disorders, such as thalassemia, sickle-cell trait, and unstable hemoglobins, give G6PD deficiency Favism rise to increased oxidative stress caused by free heme and iron generated from hemoglobin. -

Beta Thalassemia
Beta thalassemia Description Beta thalassemia is a blood disorder that reduces the production of hemoglobin. Hemoglobin is the iron-containing protein in red blood cells that carries oxygen to cells throughout the body. In people with beta thalassemia, low levels of hemoglobin lead to a lack of oxygen in many parts of the body. Affected individuals also have a shortage of red blood cells ( anemia), which can cause pale skin, weakness, fatigue, and more serious complications. People with beta thalassemia are at an increased risk of developing abnormal blood clots. Beta thalassemia is classified into two types depending on the severity of symptoms: thalassemia major (also known as Cooley's anemia) and thalassemia intermedia. Of the two types, thalassemia major is more severe. The signs and symptoms of thalassemia major appear within the first 2 years of life. Children develop life-threatening anemia. They do not gain weight and grow at the expected rate (failure to thrive) and may develop yellowing of the skin and whites of the eyes (jaundice). Affected individuals may have an enlarged spleen, liver, and heart, and their bones may be misshapen. Some adolescents with thalassemia major experience delayed puberty. Many people with thalassemia major have such severe symptoms that they need frequent blood transfusions to replenish their red blood cell supply. Over time, an influx of iron-containing hemoglobin from chronic blood transfusions can lead to a buildup of iron in the body, resulting in liver, heart, and hormone problems. Thalassemia intermedia is milder than thalassemia major. The signs and symptoms of thalassemia intermedia appear in early childhood or later in life. -

Beta- Thalassemia Major
Finally, there will be iron overload after a couple of years of transfusion. This is a An Introduction major complication of transfusions and requires removal of the iron with medication. (See our Iron Overload pamphlet for more information.) What things should I look out for? The most important things to look for include: • Your child’s color (Is he or she pale?) • If your child is gaining weight • If your child’s appetite is decreasing • If his or her belly looks bigger Published by the Cooley’s Anemia • If your child is crying a lot Foundation, 330 to • Irritability Seventh Ave., #900, • Poor growth New York, NY 10001 • Increased sleepiness/fatigue www.cooleysanemia.org Beta- (800) 522-7222. This • Increased infections publication is made • Anything else that is out of the ordinary. possible by an unrestricted Thalassemia Always call your treatment center with any questions or educational grant from concerns. NO question is a dumb one! Novartis Pharmaceuticals. Major Where can I turn for help? The Cooley’s Anemia Foundation is always ready to provide you with the information you need to deal with thalassemia, including information on many of the complications associated with thalassemia. Please contact us at (800) 522- 7222 or [email protected]. The information in this The Thalassemia Centers of Excellence have the most highly publication is for trained thalassemia experts in the country. They are located educational purposes only at: and is not intended to substitute for medical advice. You should not Children’s Hospital Boston use this information to Children’s Hospital Los Angeles diagnose or treat a health problem or disease Children’s Hospital Oakland without consulting a Children’s Hospital of Philadelphia qualified health care provider.