Onsequences of Rainforest Fragmentation for Frugivorous Vertebrates and Seed Dispersal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Papua New Guinea Huon Peninsula Extension 26Th June to 1St July 2018 (6 Days) Trip Report
Papua New Guinea Huon Peninsula Extension 26th June to 1st July 2018 (6 days) Trip Report Pesquet’s Parrots by Sue Wright Tour Leader: Adam Walleyn Rockjumper Birding Tours View more tours to Papua New Guinea Trip Report – RBL Papua New Guinea - Huon Peninsula Extension I 2018 2 Tour Summary This was our inaugural Huon Peninsula Extension. Most of the group started out with a quick flight from Moresby into Nadzab Airport. Upon arrival, we drove to our comfortable hotel on the outskirts of Lae City. After getting settled in, we set off on a short but very productive bird walk around the hotel’s expansive grounds. The best thing about the walk was how confiding the birds were –they are clearly not hunted much around here! Red-cheeked Parrot, Coconut Lorikeet, Orange-bellied Fruit Dove, Torresian Imperial Pigeon, White-bellied Cuckooshrike, Yellow-faced Myna, and Singing Starling all vied for our attention right in the parking lot. As we took a short wander, we added Hooded Butcherbird, New Guinea Friarbird and look-alike Brown Oriole, and Black and Olive-backed Sunbirds to our growing tally. A Buff-faced Pygmy Parrot zipped overhead providing just a quick view, but the highlight of the walk was clearly the Palm Cockatoo that sat out feeding contentedly on fruits – admittedly a bit of a surprise to find this species so close to a major urban centre! We were relieved when Sue had arrived and Pinon’s Imperial Pigeon by Markus Lilje joined us for dinner to complete the group! The real adventure began early the next morning, with a drive back to the airport where we were to board our flight into the Huon. -

Status of Research on Rattans: a Review
http://sciencevision.info Sci Vis 10 (2), 51-56 Research Review April-June, 2010 ISSN 0975-6175 Status of research on rattans: a review Lalnuntluanga1*, L. K. Jha2 and H. Lalramnghinglova1 1 Department of Environmental Science, Mizoram University, Aizawl 796009, India 1 Department of Environmental Science, North-Eastern Hill University, Shillong 793022, India Received 20 July 2010 | Accepted 28 July 2010 ABSTRACT Rattan forms one of the major biotic components in tropical and sub -tropical forest ecosys- tem. Contributions made by the researchers on the distribution, taxonomy and uses of rattan species in the world with special reference to India are reviewed here. Key words: Rattan; distribution; taxonomy; utilisation; N.E. states. INTRODUCTION Argentina, the Caribbean, Africa and South-East Asian regions. Rattan diversity is rich in Malay- The name ‘cane’ (rattan) stands collectively sia, Indonesia, Philippines, China, Bangladesh, for the climbing members of a big group of Sri Lanka, Myanmar and India. Rattan is of palms known as Lepidocaryoideae, fruit bearing great economic importance in handicraft and scales. Rattans/canes are prickly climbing palms furniture making because of its richness in fibre, with solid stems, belonging to the family Areca- with suitable toughness and easy for processing. ceae and the sub-family Calamoideae. They are The innumerable pinnate leaves, which extend scaly-fruited palms. The rattans/canes comprise up to two metres in length, with their mosaic more than fifty per cent of the total palm taxa arrangement play a major role in intercepting found in India.1 They are distributed throughout the splash effect of rains and improve the water South-East Asia, the Western Pacific and in the holding capacity of the soil. -

Brooklyn, Cloudland, Melsonby (Gaarraay)
BUSH BLITZ SPECIES DISCOVERY PROGRAM Brooklyn, Cloudland, Melsonby (Gaarraay) Nature Refuges Eubenangee Swamp, Hann Tableland, Melsonby (Gaarraay) National Parks Upper Bridge Creek Queensland 29 April–27 May · 26–27 July 2010 Australian Biological Resources Study What is Contents Bush Blitz? Bush Blitz is a four-year, What is Bush Blitz? 2 multi-million dollar Abbreviations 2 partnership between the Summary 3 Australian Government, Introduction 4 BHP Billiton and Earthwatch Reserves Overview 6 Australia to document plants Methods 11 and animals in selected properties across Australia’s Results 14 National Reserve System. Discussion 17 Appendix A: Species Lists 31 Fauna 32 This innovative partnership Vertebrates 32 harnesses the expertise of many Invertebrates 50 of Australia’s top scientists from Flora 62 museums, herbaria, universities, Appendix B: Threatened Species 107 and other institutions and Fauna 108 organisations across the country. Flora 111 Appendix C: Exotic and Pest Species 113 Fauna 114 Flora 115 Glossary 119 Abbreviations ANHAT Australian Natural Heritage Assessment Tool EPBC Act Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth) NCA Nature Conservation Act 1992 (Queensland) NRS National Reserve System 2 Bush Blitz survey report Summary A Bush Blitz survey was conducted in the Cape Exotic vertebrate pests were not a focus York Peninsula, Einasleigh Uplands and Wet of this Bush Blitz, however the Cane Toad Tropics bioregions of Queensland during April, (Rhinella marina) was recorded in both Cloudland May and July 2010. Results include 1,186 species Nature Refuge and Hann Tableland National added to those known across the reserves. Of Park. Only one exotic invertebrate species was these, 36 are putative species new to science, recorded, the Spiked Awlsnail (Allopeas clavulinus) including 24 species of true bug, 9 species of in Cloudland Nature Refuge. -

Bruxner Park Flora Reserve Working Plan
Bruxner Park Flora Reserve Working Plan Working Plan for Bruxner Park Flora Reserve No 3 Upper North East Forest Agreement Region North East Region Contents Page 1. DETAILS OF THE RESERVE 2 1.1 Introduction 2 1.2 Location 2 1.3 Key Attributes of the Reserve 2 1.4 General Description 2 1.5 History 6 1.6 Current Usage 8 2. SYSTEM OF MANAGEMENT 9 2.1 Objectives of Management 9 2.2 Management Strategies 9 2.3 Management Responsibility 11 2.4 Monitoring, Reporting and Review 11 3. LIST OF APPENDICES 11 Appendix 1 Map 1 Locality Appendix 1 Map 2 Cadastral Boundaries, Forest Types and Streams Appendix 1 Map 3 Vegetation Growth Stages Appendix 1 Map 4 Existing Occupation Permits and Recreation Facilities Appendix 2 Flora Species known to occur in the Reserve Appendix 3 Fauna records within the Reserve Y:\Tourism and Partnerships\Recreation Areas\Orara East SF\Bruxner Flora Reserve\FlRWP_Bruxner.docx 1 Bruxner Park Flora Reserve Working Plan 1. Details of the Reserve 1.1 Introduction This plan has been prepared as a supplementary plan under the Nature Conservation Strategy of the Upper North East Ecologically Sustainable Forest Management (ESFM) Plan. It is prepared in accordance with the terms of section 25A (5) of the Forestry Act 1916 with the objective to provide for the future management of that part of Orara East State Forest No 536 set aside as Bruxner Park Flora Reserve No 3. The plan was approved by the Minister for Forests on 16.5.2011 and will be reviewed in 2021. -

PLANT COMMUNITY FIELD GUIDE Introduction to Rainforest
PLANT COMMUNITY FIELD GUIDE Introduction to Rainforest Communities Table of Contents (click to go to page) HCCREMS Mapping ....................................................................... 3 Field Data Sheet ............................................................................. 4 Which of the following descriptions best describes your site? ................................................................ 5 Which plant community is it? .......................................................... 9 Rainforest communities of the Lower Hunter .................................. 11 Common Rainforest Species of the Lower Hunter ........................................................................ 14 A picture guide to common rainforest species of the Lower Hunter ........................................................... 17 Weeding of Rainforest Remnants ................................................... 25 Rainforest Regeneration near Black Jacks Point ............................ 27 Protection of Rainforest Remnants in the Lower Hunter & the Re-establishment of Diverse, Indigenous Plant Communities ... 28 Guidelines for a rainforest remnant planting program ..................... 31 Threatened Species ....................................................................... 36 References ..................................................................................... 43 Acknowledgements......................................................................... 43 Image Credits ................................................................................ -

A Review of Alocasia (Araceae: Colocasieae) for Thailand Including a Novel Species and New Species Records from South-West Thailand
THAI FOR. BULL. (BOT.) 36: 1–17. 2008. A review of Alocasia (Araceae: Colocasieae) for Thailand including a novel species and new species records from South-West Thailand PETER C. BOYCE* ABSTRACT. A review of Alocasia in Thailand is presented. One new species (A. hypoleuca) and three new records (A. acuminata, A. hypnosa & A. perakensis) are reported. A key to Alocasia in Thailand is presented and the new species is illustrated. INTRODUCTION Alocasia is a genus of in excess of 100 species of herbaceous, laticiferous, diminutive to gigantic, usually robust herbs. The genus has recently been revised for New Guinea (Hay, 1990), Australasia (Hay & Wise, 1991), West Malesia and Sulawesi (Hay, 1998), the Philippines (Hay, 1999) while post main-treatment novelties have been described for New Guinea (Hay, 1994) Borneo (Hay, Boyce & Wong, 1997; Hay, 2000; Boyce, 2007) & Sulawesi (Yuzammi & Hay, 1998). Currently the genus is least well understood in the trans-Himalaya (NE India to SW China) including the northern parts of Burma, Thailand, Lao PDR and Vietnam with only the flora of Bhutan (Noltie, 1994) partly covering this range. In the absence of extensive fieldwork the account presented here for Thailand can at best be regarded as provisional. STRUCTURE & TERMINOLOGY Alocasia plants are often complex in vegetative and floral structure and some notes on their morphology (based here substantially on Hay, 1998) are useful to aid identification. The stem of Alocasia, typically of most Araceae, is a physiognomically unbranched sympodium. The number of foliage leaves per module is variable between and within species and individuals, but during flowering episodes in some species it may be reduced to one. -

Extreme Ecological Specialization in a Rainforest Mammal, the Bornean
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.03.233999; this version posted August 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 2 3 4 Extreme ecological specialization in a rainforest mammal, 5 the Bornean tufted ground squirrel, Rheithrosciurus macrotis 6 7 8 Andrew J. Marshall1*, Erik Meijaard2, and Mark Leighton3 9 10 1Department of Anthropology, Department of Ecology and Evolutionary Biology, Program in the 11 Environment, and School for Environment and Sustainability, 101 West Hall, 1085 S. University 12 Ave, Ann Arbor, Michigan, 48109 USA. 13 2Borneo Futures, Block C, Unit C8, Second Floor, Lot 51461, Kg Kota Batu, Mukim Kota Batu, 14 BA 2711, Brunei Darussalam. 15 3Harvard University, 11 Divinity Ave, Cambridge, MA, 02138, U.S.A. 16 17 * Corresponding author 18 E-mail: [email protected] (AJM) 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.03.233999; this version posted August 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 19 Abstract 20 The endemic Bornean tufted ground squirrel, Rheithrosciurus macrotis, has attracted great 21 interest among biologists and the public recently. Nevertheless, we lack information on the most 22 basic aspects of its biology. -

In China: Phylogeny, Host Range, and Pathogenicity
Persoonia 45, 2020: 101–131 ISSN (Online) 1878-9080 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE https://doi.org/10.3767/persoonia.2020.45.04 Cryphonectriaceae on Myrtales in China: phylogeny, host range, and pathogenicity W. Wang1,2, G.Q. Li1, Q.L. Liu1, S.F. Chen1,2 Key words Abstract Plantation-grown Eucalyptus (Myrtaceae) and other trees residing in the Myrtales have been widely planted in southern China. These fungal pathogens include species of Cryphonectriaceae that are well-known to cause stem Eucalyptus and branch canker disease on Myrtales trees. During recent disease surveys in southern China, sporocarps with fungal pathogen typical characteristics of Cryphonectriaceae were observed on the surfaces of cankers on the stems and branches host jump of Myrtales trees. In this study, a total of 164 Cryphonectriaceae isolates were identified based on comparisons of Myrtaceae DNA sequences of the partial conserved nuclear large subunit (LSU) ribosomal DNA, internal transcribed spacer new taxa (ITS) regions including the 5.8S gene of the ribosomal DNA operon, two regions of the β-tubulin (tub2/tub1) gene, plantation forestry and the translation elongation factor 1-alpha (tef1) gene region, as well as their morphological characteristics. The results showed that eight species reside in four genera of Cryphonectriaceae occurring on the genera Eucalyptus, Melastoma (Melastomataceae), Psidium (Myrtaceae), Syzygium (Myrtaceae), and Terminalia (Combretaceae) in Myrtales. These fungal species include Chrysoporthe deuterocubensis, Celoporthe syzygii, Cel. eucalypti, Cel. guang dongensis, Cel. cerciana, a new genus and two new species, as well as one new species of Aurifilum. These new taxa are hereby described as Parvosmorbus gen. -
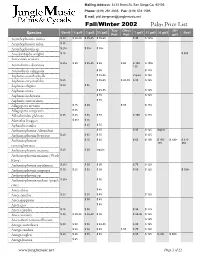
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

WIAD CONSERVATION a Handbook of Traditional Knowledge and Biodiversity
WIAD CONSERVATION A Handbook of Traditional Knowledge and Biodiversity WIAD CONSERVATION A Handbook of Traditional Knowledge and Biodiversity Table of Contents Acknowledgements ...................................................................................................................... 2 Ohu Map ...................................................................................................................................... 3 History of WIAD Conservation ...................................................................................................... 4 WIAD Legends .............................................................................................................................. 7 The Story of Julug and Tabalib ............................................................................................................... 7 Mou the Snake of A’at ........................................................................................................................... 8 The Place of Thunder ........................................................................................................................... 10 The Stone Mirror ................................................................................................................................. 11 The Weather Bird ................................................................................................................................ 12 The Story of Jelamanu Waterfall ......................................................................................................... -

Western Ghats & Sri Lanka Biodiversity Hotspot
Ecosystem Profile WESTERN GHATS & SRI LANKA BIODIVERSITY HOTSPOT WESTERN GHATS REGION FINAL VERSION MAY 2007 Prepared by: Kamal S. Bawa, Arundhati Das and Jagdish Krishnaswamy (Ashoka Trust for Research in Ecology & the Environment - ATREE) K. Ullas Karanth, N. Samba Kumar and Madhu Rao (Wildlife Conservation Society) in collaboration with: Praveen Bhargav, Wildlife First K.N. Ganeshaiah, University of Agricultural Sciences Srinivas V., Foundation for Ecological Research, Advocacy and Learning incorporating contributions from: Narayani Barve, ATREE Sham Davande, ATREE Balanchandra Hegde, Sahyadri Wildlife and Forest Conservation Trust N.M. Ishwar, Wildlife Institute of India Zafar-ul Islam, Indian Bird Conservation Network Niren Jain, Kudremukh Wildlife Foundation Jayant Kulkarni, Envirosearch S. Lele, Centre for Interdisciplinary Studies in Environment & Development M.D. Madhusudan, Nature Conservation Foundation Nandita Mahadev, University of Agricultural Sciences Kiran M.C., ATREE Prachi Mehta, Envirosearch Divya Mudappa, Nature Conservation Foundation Seema Purshothaman, ATREE Roopali Raghavan, ATREE T. R. Shankar Raman, Nature Conservation Foundation Sharmishta Sarkar, ATREE Mohammed Irfan Ullah, ATREE and with the technical support of: Conservation International-Center for Applied Biodiversity Science Assisted by the following experts and contributors: Rauf Ali Gladwin Joseph Uma Shaanker Rene Borges R. Kannan B. Siddharthan Jake Brunner Ajith Kumar C.S. Silori ii Milind Bunyan M.S.R. Murthy Mewa Singh Ravi Chellam Venkat Narayana H. Sudarshan B.A. Daniel T.S. Nayar R. Sukumar Ranjit Daniels Rohan Pethiyagoda R. Vasudeva Soubadra Devy Narendra Prasad K. Vasudevan P. Dharma Rajan M.K. Prasad Muthu Velautham P.S. Easa Asad Rahmani Arun Venkatraman Madhav Gadgil S.N. Rai Siddharth Yadav T. Ganesh Pratim Roy Santosh George P.S. -

1 History of Vitaceae Inferred from Morphology-Based
HISTORY OF VITACEAE INFERRED FROM MORPHOLOGY-BASED PHYLOGENY AND THE FOSSIL RECORD OF SEEDS By IJU CHEN A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2009 1 © 2009 Iju Chen 2 To my parents and my sisters, 2-, 3-, 4-ju 3 ACKNOWLEDGMENTS I thank Dr. Steven Manchester for providing the important fossil information, sharing the beautiful images of the fossils, and reviewing the dissertation. I thank Dr. Walter Judd for providing valuable discussion. I thank Dr. Hongshan Wang, Dr. Dario de Franceschi, Dr. Mary Dettmann, and Dr. Peta Hayes for access to the paleobotanical specimens in museum collections, Dr. Kent Perkins for arranging the herbarium loans, Dr. Suhua Shi for arranging the field trip in China, and Dr. Betsy R. Jackes for lending extant Australian vitaceous seeds and arranging the field trip in Australia. This research is partially supported by National Science Foundation Doctoral Dissertation Improvement Grants award number 0608342. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS ...............................................................................................................4 LIST OF TABLES...........................................................................................................................9 LIST OF FIGURES .......................................................................................................................11 ABSTRACT...................................................................................................................................14