Clinical and Immunological Findings in Large B-Cell Chronic Lymphocytic Leukemia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

B- and T-Cell Neoplasm Features and Fine Points
B- and T-cell neoplasm features and fine points Karen Lusky June 2021—A case of monoclonal B-cell lymphocytosis and a tour of B- and T-cell morphologies were at the heart of a CAP20 virtual presentation on neoplastic lymphocytosis. Kyle Bradley, MD, associate professor of hematopathology and director of surgical pathology at Emory University, spoke last fall on reactive (CAP TODAY, May 2021) and neoplastic lymphocytosis, with Olga Pozdnyakova, MD, PhD, who addressed neutrophilia and monocytosis (CAP TODAY, February and March 2021). Together they took attendees through a morphology-based approach to hematopoietic neoplasms presenting with an abnormal WBC differential. “Some malignant lymphocytes can closely resemble normal cells,” Dr. Bradley said in a recent interview. “The classic example would be chronic lymphocytic leukemia because individual lymphocytes in that neoplasm can look very similar to normal lymphocytes.” Dr. Bradley shared the case of a 59-year-old man with a clonal B-cell population identified by flow cytometry. It was positive for CD19, CD20, CD5, CD23, CD200, and Kappa, and negative for CD10, FMC7, and Lambda. “This is a typical phenotype for chronic lymphocytic leukemia/small lymphocytic lymphoma,” he said. However, “the blood smear is not what you typically think of for CLL.” (Fig. 1). The differential diagnosis is CLL versus monoclonal B-cell lymphocytosis, he said. In the current and previous WHO classification, CLL requires greater than 5.0 × 109/L clonal B cells. Lower counts are acceptable if there are signs or symptoms of lymphoma. Monoclonal B-cell lymphocytosis is defined as ≤ 5.0 × 109/L clonal B cells in otherwise healthy subjects. -

Burkitt Lymphoma
Board Review- Part 2B: Malignant HemePath 4/25/2018 Small Lymphocytic Lymphoma SLL: epidemiology SLL: 6.7% of non-Hodgkin lymphoma. Majority of patients >50 y/o (median 65). M:F ratio 2:1. Morphology • Lymph nodes – Effacement of architecture, pseudofollicular pattern of pale areas of large cells in a dark background of small cells. Occasionally is interfollicular. – The predominant cell is a small lymphocyte with clumped chromatin, round nucleus, ocassionally a nucleolus. – Mitotic activity usually very low. Morphology - Pseudofollicles or proliferation centers contain small, medium and large cells. - Prolymphocytes are medium-sized with dispersed chromatin and small nucleoli. - Paraimmunoblasts are medium to large cells with round to oval nuclei, dispersed chromatin, central eosinophilic nucleoli and slightly basophilic cytoplasm. Small Lymphocytic Lymphoma Pseudo-follicle Small Lymphocytic Lymphoma Prolymphocyte Paraimmunoblast Immunophenotype Express weak or dim surface IgM or IgM and IgD, CD5, CD19, CD20 (weak), CD22 (weak), CD79a, CD23, CD43, CD11c (weak). CD10-, cyclin D1-. FMC7 and CD79b negative or weak. Immunophenotype Cases with unmutated Ig variable region genes are reported to be CD38+ and ZAP70+. Immunophenotype Cytoplasmic Ig is detectable in about 5% of the cases. CD5 and CD23 are useful in distinguishing from MCL. Rarely CLL is CD23-. Rarely MCL is CD23+. Perform Cyclin D1 in CD5+/CD23- cases. Some cases with typical CLL morphology may have a different profile (CD5- or CD23-, FMC7+ or CD11c+, or strong sIg, or CD79b+). Genetics Antigen receptor genes: Ig heavy and light chain genes are rearranged. Suggestion of 2 distinct types of SLL defined by the mutational status of the IgVH genes: 40-50% show no somatic mutations of their variable region genes (naïve cells, unmutated). -
10 11 Cyto Slides 81-85
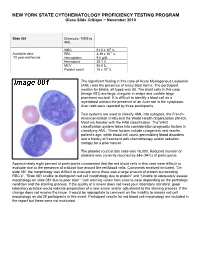
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TESTING PROGRAM Glass Slide Critique ~ November 2010 Slide 081 Diagnosis: MDS to AML 9 WBC 51.0 x 10 /L 12 Available data: RBC 3.39 x 10 /L 72 year-old female Hemoglobin 9.6 g/dL Hematocrit 29.1 % MCV 86.0 fL Platelet count 16 x 109 /L The significant finding in this case of Acute Myelogenous Leukemia (AML) was the presence of many blast forms. The participant median for blasts, all types was 88. The blast cells in this case (Image 081) are large, irregular in shape and contain large prominent nucleoli. It is difficult to identify a blast cell as a myeloblast without the presence of an Auer rod in the cytoplasm. Auer rods were reported by three participants. Two systems are used to classify AML into subtypes, the French- American-British (FAB) and the World Health Organization (WHO). Most are familiar with the FAB classification. The WHO classification system takes into consideration prognostic factors in classifying AML. These factors include cytogenetic test results, patient’s age, white blood cell count, pre-existing blood disorders and a history of treatment with chemotherapy and/or radiation therapy for a prior cancer. The platelet count in this case was 16,000. Reduced number of platelets was correctly reported by 346 (94%) of participants. Approximately eight percent of participants commented that the red blood cells in this case were difficult to evaluate due to the presence of a bluish hue around the red blood cells. Comments received included, “On slide 081 the morphology was difficult to evaluate since there was a large amount of protein surrounding RBC’s”, “Slide 081 unable to distinguish red cell morphology due to protein” and “Unable to adequately assess morphology on slide 081 due to poor stain”. -

Review Article Lymphocyte Differentiation, Repertoire Development and Migration: the Need for Mathematical Models
Comput. Math. Applic. Vol. 14, No. 9-12, pp. 657-697, 1987 0097-4943/87 $3.00+0.00 Printed in Great Britain Pergamon Journals Ltd REVIEW ARTICLE LYMPHOCYTE DIFFERENTIATION, REPERTOIRE DEVELOPMENT AND MIGRATION: THE NEED FOR MATHEMATICAL MODELS J. R. LUMB Department of Biology, Atlanta University, Atlanta, GA 30314, U.S.A. INTRODUCTION The immune system is a tremendously complicated and important system, whose central players, the lymphocytes, are able to recognize specific foreign invaders (antigens) with their specific cell surface receptors (immunoglobulins on B-lymphocytes and T cell receptors on T-lymphocytes). A number of different subpopulations of lymphocytes assume the various functions of the immune response: B cell/plasma cell production of specific immunoglobulin, helper/inducer T cells, suppressor T cells and specific cytotoxic T cells. Each of these lymphocyte subpopulations is composed of a large number of clones which each respond to specific antigens via their cell surface receptors. The study of lymphocyte kinetics involves cellular proliferation and differentiation under two different conditions: (1) the initial generation of the repertoire of lymphocytes, each of which displays a specific cell surface receptor, occurs in the absence of triggering by antigen; and (2) the specific clonal lymphocytic proliferation and differentiation occurs in response to antigen recog- nition by these receptors. Both processes involve considerations of cell cycle kinetics and population dynamics, and neither is completely understood. One approach to the study of complex systems, along with the experimental approach, is to construct mathematical models of the processes. Most of the mathematical models of the immune system have focused on the second phase, the antigen-induced activities (for reviews, see Refs [1-6]). -

Lymphopoiesis Cells and Suppresses the Earliest Events in Estrogen
Soluble Frizzled-Related Protein 1 Is Estrogen Inducible in Bone Marrow Stromal Cells and Suppresses the Earliest Events in Lymphopoiesis This information is current as of October 1, 2021. Takafumi Yokota, Kenji Oritani, Karla P. Garrett, Taku Kouro, Makoto Nishida, Isao Takahashi, Michiko Ichii, Yusuke Satoh, Paul W. Kincade and Yuzuru Kanakura J Immunol 2008; 181:6061-6072; ; doi: 10.4049/jimmunol.181.9.6061 Downloaded from http://www.jimmunol.org/content/181/9/6061 References This article cites 67 articles, 27 of which you can access for free at: http://www.jimmunol.org/content/181/9/6061.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on October 1, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2008 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Soluble Frizzled-Related Protein 1 Is Estrogen Inducible in Bone Marrow Stromal Cells and Suppresses the Earliest Events in Lymphopoiesis1 Takafumi Yokota,2* Kenji Oritani,* Karla P. -

Fetal Lymphoid Progenitors Become Restricted to B-1 Fates Coincident with IL- 7Rα Expression
RESEARCH ARTICLE Fetal Lymphoid Progenitors Become Restricted to B-1 Fates Coincident with IL- 7Rα Expression Ryuji Iida1,2*, Kaori Shinoda1, Yuki Hayano1, Yoshinori Nagai3,4, Kiyoshi Takatsu3,5, Taku Kouro1 1 Laboratory of Immune Modulation, National Institutes of Biomedical Innovation, Health and Nutrition, Ibaraki, Osaka, Japan, 2 Department of Cell Cultures, National Institutes of Biomedical Innovation, Health and Nutrition, Ibaraki, Osaka, Japan, 3 Department of Immunobiology and Pharmacological Genetics, a11111 University of Toyama, Toyama-shi, Toyama, Japan, 4 JST, PRESTO, Kawaguchi, Saitama, Japan, 5 Toyama Prefectural Institute for Pharmaceutical Research, Imizu City, Toyama, Japan * [email protected] Abstract OPEN ACCESS B-1 cells represent a sub-fraction of B lymphocytes that participate in T cell-independent Citation: Iida R, Shinoda K, Hayano Y, Nagai Y, Takatsu K, Kouro T (2016) Fetal Lymphoid antibody production and contribute to innate immunity. While the production of B-1 cells is Progenitors Become Restricted to B-1 Fates favored during the fetal waves of lymphopoiesis, it has been unclear when and how that dif- Coincident with IL-7Rα Expression. PLoS ONE 11 ferentiation option is specified. To clarify this, lymphoid and hematopoietic progenitors of (10): e0165676. doi:10.1371/journal. pone.0165676 fetal liver (FL) and adult bone marrow (ABM) were examined for the B cell differentiation potential. Mouse common lymphoid progenitors (CLPs) and more primitive KSL fraction of Editor: Connie J Eaves, B.C. Cancer Agency, CANADA FL and ABM were transferred to SCID mice and donor-derived B cell subsets were ana- lyzed 4 weeks later. CLPs were also cultured on ST2 stromal cells for 6 days prior to trans- Received: July 30, 2016 plantation. -

Notch Receptor Ligation Progress to DN2/3 Stage Thymocytes with Propensity of Adult Lymphoid Progenitors To
Propensity of Adult Lymphoid Progenitors to Progress to DN2/3 Stage Thymocytes with Notch Receptor Ligation This information is current as Jiaxue Huang, Karla P. Garrett, Rosana Pelayo, Juan Carlos of September 29, 2021. Zúñiga-Pflücker, Howard T. Petrie and Paul W. Kincade J Immunol 2005; 175:4858-4865; ; doi: 10.4049/jimmunol.175.8.4858 http://www.jimmunol.org/content/175/8/4858 Downloaded from References This article cites 45 articles, 20 of which you can access for free at: http://www.jimmunol.org/content/175/8/4858.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 29, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2005 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Propensity of Adult Lymphoid Progenitors to Progress to DN2/ 3 Stage Thymocytes with Notch Receptor Ligation1 Jiaxue Huang,*† Karla P. Garrett,* Rosana Pelayo,* Juan Carlos Zu´n˜iga-Pflu¨cker,‡ Howard T. -

Src Homology 2–Containing 5-Inositol Phosphatase (SHIP)
Src Homology 2–containing 5-Inositol Phosphatase (SHIP) Suppresses an Early Stage of Lymphoid Cell Development through Elevated Interleukin-6 Production by Myeloid Cells in Bone Marrow Koji Nakamura,1 Taku Kouro,1 Paul W. Kincade,1,2 Alexander Malykhin,1 Kazuhiko Maeda,1 and K. Mark Coggeshall1,2,3 1Immunobiology and Cancer Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104 2Department of Microbiology and Immunology, and 3Department of Cell Biology, University of Oklahoma, Oklahoma City, OK 73104 Abstract The Src homology (SH)2–containing inositol 5-phosphatase (SHIP) negatively regulates a variety of immune responses through inhibitory immune receptors. In SHIPϪ/Ϫ animals, we found that the number of early lymphoid progenitors in the bone marrow was significantly reduced and accompanied by expansion of myeloid cells. We exploited an in vitro system using hemato- poietic progenitors that reproduced the in vivo phenotype of SHIPϪ/Ϫ mice. Lineage-negative marrow (LinϪ) cells isolated from wild-type mice failed to differentiate into B cells when co- cultured with those of SHIPϪ/Ϫ mice. Furthermore, culture supernatants of SHIPϪ/Ϫ LinϪ cells suppressed the B lineage expansion of wild-type lineage-negative cells, suggesting the presence of a suppressive cytokine. SHIPϪ/Ϫ LinϪ cells contained more IL-6 transcripts than wild-type LinϪ cells, and neutralizing anti–IL-6 antibody rescued the B lineage expansion suppressed by the supernatants of SHIPϪ/Ϫ LinϪ cells. Finally, we found that addition of recombi- nant IL-6 to cultures of wild-type LinϪ bone marrow cells reproduced the phenotype of SHIPϪ/Ϫ bone marrow cultures: suppression of B cell development and expansion of myeloid cells. -

Evolution of Technologies for White Blood Cell Differential at HORIBA Medical
Review Review HORIBA Medical in Europe: Evolution of Technologies for White Blood Cell Differential at HORIBA Medical Philippe NERIN This article retraces the evolution of technologies for leucocyte differential, which is a key feature of hematology analyzers. It is emphasized here the way HORIBA Medical started using simple and robust technologies, based on CIS (Cell Impedance Signal) for 3 part DIFF, and then evolved toward more sophisticated bio-photonics designs including cut edge technologies, considered as pioneering works in the field of flow cytometry. This article was presented by myself at ISLH 2012, in Nice, France, at the luncheon session attended by about 200 worldwide customers. Introduction It is worth pointing out that the first attempts in automatic measurements of blood cells go back to the mid twentieth century, when two techniques were developed in parallel: impedance measurement (the so-called electronic gate) and the first optical scattering detector. Improved year after year along with specific reagents, the electrical and optical methods allowed considerable projections in the field of hematology since, in most cases, the main cellular populations (examined early back by a photonic microscope) are differentiated and taken into account with acceptable accuracy. Nowadays, a hematology analyzer is a complex system involving various disciplines and technologies such as biology, biochemistry, chemistry, fluidics, optics, electronics, data acquisition and statistical data processing.[1] Basically, a hematology analyzer is a fully automated device able to sample the blood through a hermetic stopper, dilute the sample, add a specific reagent for single or multiple cell parameters detection, carry the cells within a flow stream toward one or several sensors, acquire and store the information resulting from several physical mechanisms, and finally process the raw data by using a classification algorithm. -

And T Lymphoid Leukaemias
J Clin Pathol: first published as 10.1136/jcp.42.6.567 on 1 June 1989. Downloaded from J Clin Pathol 1989;42:567-584 Proposals for the classification of chronic (mature) B and T lymphoid leukaemias J M BENNETT,* D CATOVSKY,I M-T DANIEL,$ G FLANDRIN,$ D A G GALTON,¶ H R GRALNICK,§ C SULTAN,** THE FRENCH-AMERICAN- BRITISH (FAB) COOPERATIVE GROUP From the * University ofRochester Cancer Center, Rochester, New York, United States ofAmerica; tAcademic Department ofHaematology, The Royal Marsden Hospital, London; $Institut de Recherches sur les Leucemies et les Maladies du Sang, Hopital Saint-Louis, Paris, France; ¶MRC Leukaemia Unit, Royal Postgraduate Medical School, London; §Hematology Service, National Institutes ofHealth, Bethesda, Maryland, United States ofAmerica; **Service Central d'Hematologie-Immunologie, Hopital Henri Mondor, Creteil, France SUMMARY Peripheral blood, bone marrow films, and bone marrow biopsy specimens from 110 patients, well characterised by clinical and laboratory studies, including electron microscopy, were reviewed, to determine proposals for the classification of chronic (mature) B and T cell leukaemias. On the basis of cytology and membrane phenotype the following disorders were defined: (i) B cell type: chronic lymphocytic leukaemia (CLL); CLL ofmixed cell type, which includes cases with more than 10% and less than 55% prolymphocytes (CLL/PL), and a less well defined form with pleomorphic lymphocytes but less than 10% prolymphocytes; prolymphocytic leukaemia (PLL); copyright. hairy cell leukaemia (HCL); HCL variant; splenic lymphoma with circulating villous lymphocytes; leukaemic phase of non-Hodgkin's lymphoma (follicular lymphoma, intermediate, or mantle zone lymphoma and others); lymphoplasmacytic lymphoma with peripheral blood disease (mostly Waldenstr6m's macroglobulinaemia); and plasma cell leukaemia. -
The Identification of Mature and Immature Leucocytes in Peripheral Blood Smears and Bone Marrow Preparations Is Fundamental To
The identification of mature and immature blood cells in peripheral blood smears and bone marrow preparations is fundamental to the laboratory diagnosis of haematological disorders. Here, you may review the mature and immature white cells to gain more practice and confidence in their identification. Immature cells are found in peripheral blood in leukaemia. Modified to printer-friendly form from Quensland University of Tehcnology, Our Medical Science pages & University of California Davis School of Medicine) Granulocytes Bone Marrow Peripheral Blood (Neutrophile) Left - Nonsegmented neutrophil Myelocyte (Neutrophile) Metamyelocyte Cell size: 12 -15 -m Cell size: 10-16 -m Cell size: 12 -23 -m N:C ratio: Ratio more reduced, 40% N:C ratio: Ratio more reduced 30 - 40% N:C ratio: 60%, decreasing Cytoplasm: Similar to mature cell Cytoplasm: Similar to mature cell Cytoplasm: Development of Nucleus: Indentation of nucleus Nucleus: Curved, without distinct lobes secondary (specific) granules, some begins; heavy chromatin clumping, Note: Also referred as "band forms" or primary granules may be visible nucleoli not visible "stab cells". Nucleus: Oval or round, with further Right - Segmented Neutrophil clumping, nucleoli no long visible Cell size: 10-16 -m Note: The term myelocyte infers it is a N:C ratio: Ratio more reduced 20 - 30 % neutrophil myelocyte, also found are Cytoplasm: Fine specific granules, pink- eosinophil myelocytes and basophil tan cytoplasm. myelocytes. Nucleus: Segmented nucleus (normal up to 5 lobes) Myeloblast Promyelocyte Cell -

Morphological and Cytochemical Identification of Lymphoid Cells
77 Lymphology 10 (1977) 77-84 © Georg Thieme Verlag Stuttgart Morphological and Cytochemical Identification of Lymphoid Cells D. Catovsky, A. E~no M.R.C. Leukaemia Uriit, Royal- Postgraduate Medical School, London Summary Morphology - Light and Electron Micro This paper reviews the contribution of: light and scopy (TEM) electron microscopy, surface structure .and cyto Morphological features of lymphocytes may chemistry (acid phosphatase, B-glucuronidase, a-naphthyl esterase, and PAS reactions) in the depend on the state of transformation and on recognition and classification of normcd and ab the degree of differentiation. The latter may normal lymphocyte populations be demonstrated better by surface markers. ''Mature" lymphocytes can readily be dis tinghished from other leukocytes, but it is In recent years important advances in the not possible to distinguish between T and B · methods for re~gnition of different popu lymphocytes with.either light microscopy or lations of lymphocytes have taken place {1 ). transmission electron microscopy. Normal anti At the same time new techniques in cell mor genically stimulated lymphocytes can o:casion phology and cytochemistry have been applied ally be difficult to distinguish from malignant successfully to the study of lymphoid cells. cells. Transitional forms between B lympho The two main problems in the identification cytes and plasma cells such as seen in W~den of lymphocytes are: strom 's macroglobulinemia are characterized 1. Distinguishing lymphoid from nonlymphoid by marked basophilic cytoplasm and increased cells, particularly when the features of amounts of rough endoplasmic reticulum differentiation and maturation ~e not obvi (Fig. 1). ous. This is apparent in the problem of The importance of lymphocyte morphology diagnosing and classifying leukemias (2) and its accurate description is particularly high and lymphomas in order to es~blish with lighted in the field of non-Hodgkin lympho certainty the nature of the malignant mas.