Coffee and Its Preparation (1St Revision)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caffeine Sources and Consumption Among Saudi Adults Living with Diabetes and Its Potential Effect on Hba1c
nutrients Article Caffeine Sources and Consumption among Saudi Adults Living with Diabetes and Its Potential Effect on HbA1c Salwa Ali Albar 1,* , Merfat Abdulrahman Almaghrabi 1,* , Rawabi Ahmed Bukhari 1 , Rawan Hussein Alghanmi 1 , Maha Ali Althaiban 1 and Khaled A. Yaghmour 2 1 Food and Nutrition Department, King Abdulaziz University, P.O. Box 80200, Jeddah 21589, Saudi Arabia; [email protected] (R.A.B.); [email protected] (R.H.A.); [email protected] (M.A.A.) 2 Family Medicine Department, Faculty of Medicine, King Abdulaziz University, P.O. Box 80200, Jeddah 21589, Saudi Arabia; [email protected] * Correspondence: [email protected] (S.A.A.); [email protected] (M.A.A.); Tel.: +966-505317160 (S.A.A.); +966-504685978 (M.A.A.) Abstract: Information regarding the spread and effect of coffee and caffeine intake by individuals with type II diabetes remains unclear. This study aims to identify the amount and sources of habitual caffeine intake by individuals with type II diabetes and to investigate its association with other health outcomes, especially HbA1c. This is a cross-sectional survey involving 100 people medically defined as having type II diabetes comprising both genders, recruited from a care centre. All participants completed a caffeine semi-quantitative food frequency questionnaire (C-FFQ) to estimate their caffeine consumption, a two day 24-h recall, and a detailed questionnaire. The average caffeine intake Citation: Albar, S.A.; was calculated from all sources and the differences in mean by gender were tested using a regression Almaghrabi, M.A.; Bukhari, R.A.; model (adjusted to important confounders). -

Color Stability of Restorative Materials in Response to Arabic Coffee, Turkish Coffee and Nescafe
JCDP 10.5005/jp-journals-10024-1385 ORIGINAL RESEARCH Color Stability of Restorative Materials in Response to Arabic Coffee, Turkish Coffee and Nescafe Color Stability of Restorative Materials in Response to Arabic Coffee, Turkish Coffee and Nescafe Khalid H Al-Samadani ABSTRACT Clinical performance of dental resin composites has been Objective: To evaluate the effect of Arabic coffee, Turkish coffee significantly improved over last decades through and Nescafe on the color stability of four different composite innovations of nanofillers to produce adequate strength and resins after a period of aging time 1, 7 and 30 days. excellent wear resistance along with retaining translucency, Materials and methods: Twenty specimens from each type of development of stable polymerization promoters to enhance tested composite resin material were prepared. Five specimens color stability; reduction of polymerization shrinkage to from each tested material (Z350 XT, Artist, GC and Z250) was evaluated after storage in Arabic coffee, Turkish coffee, Nescafe improve marginal adaptation and to prevent recurrent caries. and distil water (control) at 37°C in a dark container for 1, 7 and Application of nanotechnology contributed most important 30 days. Color measurement was done using colorimeter based role since last few years. Dental resin composites with on the CIE L* a* b* color scale. Color differences E*ab, b* nanofillers (1-50 nm) showed increased strength, surface and a* among specimens immersed in distil water and staining coffee beverages were evaluated overtime. Mean values were smoothness and gloss, and decreased polymerization 3 statistically analyzed with one-way analysis of variance shrinkage while maintaining low viscosity. -

How a Few Simple Things Changed History
How a Few Simple Things Changed History Class 5 William A. Reader E-mail: [email protected] 1 1 What We Will Cover Today • The Impact of the Potato – The Irish Potato Famine & the U.S. Civil War – French Fries, Fast Food & McDonald’s • The Impact of Coffee – Notes on Coffee – Consequences of Coffee – Impact of the Coffeehouse • The Impact of Tea – Notes on Tea – Consequences of Tea 2 2 Effects of the Potato Famine - 4 • Effects of Irish Famine emigration to the U.S. – Led to the growth of political machines in northern U.S. cities – Destabilized American politics in the decade before the Civil War • Fostered a nativist anti-Catholic reaction among native Protestants that led to the creation of the Know-Nothing Party, the weakening of the Democrats, and the destruction of the Whigs – This paved the way for the rise of the Republican Party and the Civil War • Increased the relative population of the North, intensifying Southern fears of political marginalization – this in turn intensified pro-secession sentiment 3 Irish immigrants brought with them from Ireland a knowledge of the English language, a familiarity with the forms of English political institutions, and poverty. Situated in Irish ghettoes in northern big cities, they were quick to politically mobilize and vote in order to obtain help and jobs. One consequence was that in a generation, the Irish became dominant in such areas of local governance as policemen, firemen, low-level office holders, and construction. By using income from “simple” and “honest” graft, Irish political bosses (many of whom were saloon owners) bestowed jobs and largesse (such as food, coal, and Xmas turkeys) on the needy in exchange for their votes. -

Effect of Arabic and Green Coffee Beans on Lowering Lipid Profile Parameters in Male Rats
Australian Journal of Basic and Applied Sciences, 10(18) December 2016, Pages: 310-317 AUSTRALIAN JOURNAL OF BASIC AND APPLIED SCIENCES ISSN:1991-8178 EISSN: 2309-8414 Journal home page: www.ajbasweb.com Effect of Arabic and Green Coffee Beans on Lowering Lipid Profile Parameters in Male Rats Ebtihal Y. Khojah Department of Nutrition and Food Science, College of Designs and Home Economics, Taif University, Saudi Arabia Address For Correspondence: Ebtihal Y. Khojah Department of Nutrition and Food Science, College of Designs and Home Economics, Taif University, Saudi Arabia ARTICLE INFO ABSTRACT Article history: This research was carried out to evaluate the effects of using powder and extract of Received 19 September 2016 Arabic and green coffee to improve body weight, the serum total cholesterol; Accepted 10 December 2016 triglycerides, lipoprotein fractions (HDLc, LDLc and VLDLc), atherogenic index (AI) Published 31 December 2016 and leptin hormone in different groups hypercholesterolemia male rats. Caffeine, chlorogenic acid and total phenolic content were determined in Arabic and green coffee. The results showed that the green coffee had contained the highest amounts Keywords: from caffeine, chlorogenic acid and total phenolic content (2.10%, 9.74% and 366.0 Green coffee, Arabic coffee, lipid mg/100g) whereas, Arabic coffee was 1.5%, 7.80% and 206.0 mg/100g, respectively. profile, atherogenic index, leptin At the end of experimental biological (4 weeks) the resultant observed that the rats hormone. group fed orally on green coffee powder were significantly decreased in body weight and feed efficiency ratio than rats group fed orally on Arabic coffee powder. -

Business Idea for Starbucks: Organic Yerba Mate Beverages
Business Idea for Starbucks: Organic Yerba Mate Beverages Sourav Basak1, Ranjith P.V2, Preethi A. Prakash3, Ghezlan Albesis4, Majdi Anwar Quttainah5, Sonia Shukla6, Rudresh Pandey7 CMS Business School1,2,3 No.17, Seshadri Rd, Gandhi Nagar, Bengaluru, Karnataka 560009, India Kuwait University4,5 Jamal Abdul Nasser St, Kuwait ABES Engineering College6,7 Campus -1, 19th KM Stone, NH 24, Ghaziabad, Uttar Pradesh 201009, India Correspondence Email: [email protected] ABSTRACT The report here is basically a research-based business idea for Starbucks Coffee Company that operates all over the world. The idea is line with the market dynamics and the needs of the target market i.e. the masses across the world. The idea is that Starbucks should start a separate line of coffee shops in various parts of the word that will serve Yerba Mate herbal coffee, tea and Beverages. This is a famous coffee and tea in South Africa that has extensive health benefits and falls in the domain of third wave of coffee for the specialty coffee lovers. This business idea will be implemented by Starbucks as a specific niche in the coffee or restaurant industry. Now since the population of world is facing high rates of obesity, and with increasing quality consciousness and love for specialty coffee, this business idea presents great opportunity for the company. The idea is based on research using market analysis and tools like PEST analysis. Keywords: Business, Idea, Marketing, Starbucks, Tea INTRODUCTION The company operating as a multinational conglomerate in many parts of the world that has been selected for the business idea or plan is Starbucks Coffee Company. -

Effect of Green and Degree of Roasted Arabic Coffee on Hyperlipidemia and Antioxidant Status in Diabetic Rats
Advance Journal of Food Science and Technology 5(5): 619-626, 2013 ISSN: 2042-4868; e-ISSN: 2042-4876 © Maxwell Scientific Organization, 2013 Submitted: January 03, 2013 Accepted: January 31, 2013 Published: May 05, 2013 Effect of Green and Degree of Roasted Arabic Coffee on Hyperlipidemia and Antioxidant Status in Diabetic Rats 1Gaafar M. Ahmed and 2Heba E. El-Ghamery and 3Mahmuod F. Samy 1Food Technology Research Institute, Agricultural Research Center, Giza, Egypt 2Faculty of Education, King Khalid University, Saudi Arabia 3Department of Biotechnology, Faculty of Science, Taif University, Taif, Kingdom of Saudi Arabia Abstract: This study aims to examine the effects of green and different roasted degree of Arabic coffee on alloxan induced diabetes in rats. Animals were allocated into five groups of six rats each: a normal control group, diabetic group, diabetic rats fed with green Arabic coffee, diabetic rats fed with light roasted coffee and diabetic rats fed with dark roasted coffee group. The results showed increasing roasting degrees led to a decrease in moisture, radical- scavenging activity and total phenols. The diabetic rats presented a significant increase in blood glucose, plasma lipid profile compared to the control group. In addition, plasma malonaldialdehyde levels significantly increased compared to normal control group. Antioxidant enzymes activities such as superoxide dismutase and reduced Glutathione (GSH) levels significantly decreased in the plasma of diabetic rats compared to normal controls. The results showed that the experimental rats supplemented by green and roasted Arabic coffee significant increased feed efficiency ratio than diabetic control group. At the end of the study period, the experimental rats were showed significant improvement in blood glucose. -
Grocery Master List
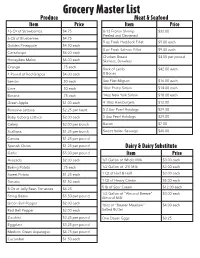
Grocery Master List Produce Meat & Seafood Item Price Item Price 16 Oz of Strawberries $4.75 8-12 Frozen Shrimp $32.00 Peeled and Deviened 6 Oz of Blueberries $4.75 9 oz Fresh Haddock Fillet $9.00 each Golden Pineapple $4.50 each 8 oz Fresh Salmon Fillet $9.00 each Cantaloupe $4.00 each Chicken Breast $4.50 per pound Honeydew Melon $6.00 each Skinless, Boneless Orange .75 each Rack of Lamb $42.00 each 1 Pound of Red Grapes $4.00 each 8 Bones Lemon .50 each 8oz Filet Mignon $16.00 each Lime .50 each 10oz Prime Sirloin $14.00 each Banana .75 each 14oz New York Sirloin $18.00 each Green Apple $1.00 each 4 10oz Hamburgers $12.00 Romaine Lettuce $2.25 per heart 5 2.6oz Pearl Hotdogs $29.00 Baby Iceberg Lettuce $2.00 each 5 4oz Pearl Hotdogs $29.00 Celery $2.00 per bunch Bacon $7.00 Scallions $1.25 per bunch Sweet Italian Sausage $20.00 Carrots $1.25 per pound Spanish Onion $1.25 per pound Dairy & Dairy Substitute Garlic $5.00 per pound Item Price Avocado $2.00 each 1/2 Gallon of Whole Milk $3.00 each Baking Potato .75 each 1/2 Gallon of 2% Milk $3.00 each Sweet Potato $1.25 each 1 Qt of Half & Half $3.00 each Tomato $1.50 each 1 Qt of Heavy Cream $5.00 each 8 Oz of Jelly Bean Tomatoes $4.25 5 lb of Sour Cream $12.00 each 1/2 Gallon of “Almond Breeze” $3.00 each String Beans $5.50 per pound Almond Milk Green Bell Pepper $2.00 each 16oz of “Beaver Meadow” $4.00 each Red Bell Pepper $2.00 each Salted Butter Zucchini $3.25 per pound One Dozen Eggs $3.25 Eggplant $3.25 per pound Medium Green Asparagus $4.75 per pound Cucumber $1.50 each Pantry -

The Promotion & Preservation of Saudi Arabia's Cultural Identity
The Promotion & Preservation of Saudi Arabia’s Cultural Identity Through Modernizing Traditional Arts & Crafts by Bodoor Hussain Alahmadi B.A. in Accounting, May 2010, King Abdulaziz University A Thesis submitted to The Faculty of The Columbian College of Arts and Sciences (formerly the Corcoran College of Arts + Design ) of The George Washington University in partial fulfillment of the requirements for the degree of Master of Art May 17, 2015 Thesis directed by Emily Bishop Mckenna Adjunct Professor of Interior Design © Copyright 2015 by Bodoor Alahmadi All Rights Reserved ii Abstract The Promotion & Preservation of Saudi Arabia’s Cultural Identity Through Modernizing Traditional Arts & Crafts This research aims to show the benefits of developing a Community Cultural Arts and Crafts Center in Jeddah, Saudi Arabia which would promote the passage of knowledge and skills of traditional arts and crafts. This proposal targets two specific demographics; senior citizens and youth . Saudi Arabia’s population is disproportionately i young. It is estimated that more than half of the population is below 20 years old . The senior citizens would be passing down their knowledge and skills with an emphasis on passing down firsthand handicraft skills to the youth of the country. This research focuses on the current need to preserve art in Saudi Arabia and create an intergenerational dialogue through handicrafts. This research emphasizes the need to reconnect with younger generations through hands on classroom experience to teach them about their history through cultural awareness classes. There are several specific art forms that this research has explored including: historical methods/changes, weaving, jewelry making, dallah coffee pot making, pottery making, carving, and calligraphy. -

How to Make Arabic Coffee
How to Make Arabic Coffee By Mr. Jamal Al-Otaibi, Saudi Arabia [email protected] Arabic Coffee is a general name that refers the way coffee is prepared in many Arabic Countries throughout the Middle East. Arabic Coffee has a Special pot called (Dallah) as well as particular coffee cup (Finjal )that are small but without handle. Dallah with Arabic Coffee Cup (Finjal) You can buy an Arabic Coffee from any Super Market . it comes as medium sacking bag in 10 Kg and 20 Kg. as a following picture ©2010 After you get a coffee you have to burn it to be brown color take care if you increase the burning you will infringe ARBIC standards. You can use regular clean pan or coffee Roaster at your kitchen but take care for the burning color it must be brown. Now grind it, the people are not same for the grinding mood some people like it very fine and some like it has little granulated. ©2010 Preparing Steps 1- Fill a little more than half of the pot with (1 Le er) of water . 2- Turn stove on to on medium heat. 3- Boil water. 4- Lower heat. 5- Add FOUR tablespoons of Arabic grind coffee to water inside coffee pot. Keep temperature low and let the coffee brew on low temperature A er 5 minutes on low heat the coffee will start to boil inside pot and starts foaming and slowly rise to top. 6- Let the coffee boil with water for 6 minutes to extract the coffee. 7- Turn stove off and let the pot settle for a minute. -

Thrombosis Preventive Potential of Chicory
Thrombosis preventive potential of chicory coffee consumption: a clinical study Edit Schumacher, Éva Vigh, Valéria Molnár, Péter Kenyeres, Gergely Fehér, Gábor Késmárky, Kálmán Tóth, János Garai To cite this version: Edit Schumacher, Éva Vigh, Valéria Molnár, Péter Kenyeres, Gergely Fehér, et al.. Thrombosis preventive potential of chicory coffee consumption: a clinical study. Phytotherapy Research, Wiley, 2011, 10.1002/ptr.3481. hal-00625153 HAL Id: hal-00625153 https://hal.archives-ouvertes.fr/hal-00625153 Submitted on 21 Sep 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Phytotherapy Research Thrombosis preventive potential of chicory coffee consumption: a clinical study Journal: Phytotherapy Research ManuscriptFor ID: PTR-10-0363.R2 Peer Review Wiley - Manuscript type: Full Paper Date Submitted by the 19-Feb-2011 Author: Complete List of Authors: Schumacher, Edit; University of Pécs, Medical School, Department of Pathophysiology and Gerontology; University of Pécs, Medical School, 1st Department of Internal Medicine Vigh, Éva; University -

Madhura Muralidharan Coffee
COFFEE AND ITS TWO COLONISATIONS Authored by: Raamesh Gowri Raghavan Edited by: Madhura Muralidharan Coffee: The first colonisation of the land The founding myth of coffee growing in India holds that in the 17th century, the Sufi mystic Hazrat Shah-Janab Alla Magatabi (popularly known as Baba Budan) established the first coffee plantations in India in the hills now known as Bababudanagiri in Chikkamagaluru district of Karnataka, after smuggling out seven unroasted coffee beans tied to his belly (at great risk to his life) from Mocha, Yemen (Sridhar, 2008). Dates are uncertain as is the historicity of this claim: the website of the Coffee Board of India picks a date of 1600 CE (“The Coffee Board, Ministry of Commerce and Industry, Government of India”, 2014) while the website of the Collectorate of Chikkamagaluru says 1670 (“History of Chikkamagaluru”, n.d.). Nevertheless, the fact remains that Europeans did find Coffea arabica growing in India. The first European reference to coffee in the Indian peninsula comes from 1723 (Mohandas, 2014). The complaint of the taste not being “refined” stems from the fact that Indian coffee was shipped to Arabia and remarketed as Mocha to the Europeans (Sridhar, 2008). The second report comes from one Francis Buchanan from Kerala that one box was the total export of coffee from Kannur in 1799 and 6 chests and 6 mounds in 1800 (Mohandas, 2014). Before the 1830s, coffee was cultivated mostly in Mudigere, Koppa, Manjarabad, Sakaleshpur and Kadur in the much-reduced Mysore state (newly restored to the Wodeyars) (Sridhar, 2008) and in Anjarakkandy in Wayanad district of Kerala (Mohandas, 2014) as a hittalu (backyard) crop by the peasantry. -

Turkish Cultural Heritage: a Cup of Coffee
Accepted Manuscript Turkish cultural heritage: A cup of coffee Birsen Yılmaz, Nilüfer Acar-Tek, Saniye Sözlü PII: S2352-6181(17)30184-1 DOI: 10.1016/j.jef.2017.11.003 Reference: JEF 136 To appear in: Journal of Ethnic Foods Received Date: 13 October 2017 Revised Date: 7 November 2017 Accepted Date: 8 November 2017 Please cite this article as: Yılmaz B, Acar-Tek N, Sözlü S, Turkish cultural heritage: A cup of coffee, Journal of Ethnic Foods (2017), doi: 10.1016/j.jef.2017.11.003. This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. ACCEPTED MANUSCRIPT Turkish cultural heritage: A cup of coffee Running Title: Turkish coffee and its culture Birsen Yılmaz 1*, Nilüfer Acar-Tek 2, Saniye Sözlü 3 1Gazi University Faculty of Health Sciences, Department of Nutrition and Dietetics, Ankara/Turkey, Tel: +90 312 2162968, Fax: +90 312 2162636, E-mail: [email protected] ORCID ID: 0000-0002-4866-2818 Link: http://orcid.org/0000-0002-4866-2818 2Gazi University Faculty of Health Sciences, Department of Nutrition and Dietetics, Ankara/Turkey, Tel: +90 312 2162603, Fax: +90 312 2162636, E-mail: [email protected] 3Gazi University Faculty of Health Sciences,MANUSCRIPT Department of Nutrition and Dietetics, Ankara/Turkey, Tel: +90 312 2162968, Fax: +90 312 2162636, E-mail: [email protected] Word count: 4481 ACCEPTED ACCEPTED MANUSCRIPT Turkish cultural heritage: A cup of coffee Abstract Setting out a fabulous journey from a tiny bean, coffee is the stimulant of heart and mind and a mysterious plant which stiffens friendship and takes your tiredness away during the day.