Iridescent Colouration of Male Anna's Hummingbird
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
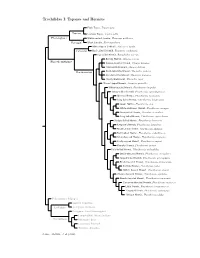
Topazes and Hermits
Trochilidae I: Topazes and Hermits Fiery Topaz, Topaza pyra Topazini Crimson Topaz, Topaza pella Florisuginae White-necked Jacobin, Florisuga mellivora Florisugini Black Jacobin, Florisuga fusca White-tipped Sicklebill, Eutoxeres aquila Eutoxerini Buff-tailed Sicklebill, Eutoxeres condamini Saw-billed Hermit, Ramphodon naevius Bronzy Hermit, Glaucis aeneus Phaethornithinae Rufous-breasted Hermit, Glaucis hirsutus ?Hook-billed Hermit, Glaucis dohrnii Threnetes ruckeri Phaethornithini Band-tailed Barbthroat, Pale-tailed Barbthroat, Threnetes leucurus ?Sooty Barbthroat, Threnetes niger ?Broad-tipped Hermit, Anopetia gounellei White-bearded Hermit, Phaethornis hispidus Tawny-bellied Hermit, Phaethornis syrmatophorus Mexican Hermit, Phaethornis mexicanus Long-billed Hermit, Phaethornis longirostris Green Hermit, Phaethornis guy White-whiskered Hermit, Phaethornis yaruqui Great-billed Hermit, Phaethornis malaris Long-tailed Hermit, Phaethornis superciliosus Straight-billed Hermit, Phaethornis bourcieri Koepcke’s Hermit, Phaethornis koepckeae Needle-billed Hermit, Phaethornis philippii Buff-bellied Hermit, Phaethornis subochraceus Scale-throated Hermit, Phaethornis eurynome Sooty-capped Hermit, Phaethornis augusti Planalto Hermit, Phaethornis pretrei Pale-bellied Hermit, Phaethornis anthophilus Stripe-throated Hermit, Phaethornis striigularis Gray-chinned Hermit, Phaethornis griseogularis Black-throated Hermit, Phaethornis atrimentalis Reddish Hermit, Phaethornis ruber ?White-browed Hermit, Phaethornis stuarti ?Dusky-throated Hermit, Phaethornis squalidus Streak-throated Hermit, Phaethornis rupurumii Cinnamon-throated Hermit, Phaethornis nattereri Little Hermit, Phaethornis longuemareus ?Tapajos Hermit, Phaethornis aethopygus ?Minute Hermit, Phaethornis idaliae Polytminae: Mangos Lesbiini: Coquettes Lesbiinae Coeligenini: Brilliants Patagonini: Giant Hummingbird Lampornithini: Mountain-Gems Tro chilinae Mellisugini: Bees Cynanthini: Emeralds Trochilini: Amazilias Source: McGuire et al. (2014).. -

Do Hummingbirds See in Ultraviolet?
The Open Medical Informatics Journal, 2009, 3, 9-12 9 Open Access Do Hummingbirds See in Ultraviolet? M. Curé*,1 and A.G. Palacios2 1Departamento de Física y Astronomía, Facultad de Ciencias, Universidad de Valparaíso, Chile 2Centro de Neurociencia de Valparaíso, Facultad de Ciencias, Universidad de Valparaíso, Chile Abstract: We present a numerical model to fit the electroretinogram (ERG), a gross evoked eye visual potential, that originate in the retina through photons absorption by photoreceptors and then involve the contribution form others retinal neurons. We use the ERG measured in a hummingbird, to evaluate the most likely retinal mechanism - cones visual pig- ments and oil-droplets - that participate in their high dimensional tetra or pentachromatic color hyperspace. The model - a nonlinear fit - appears to be a very useful tool to predict the underlying contribution visual mechanism for a variety of retinal preparation. Keywords: Color vision, electroretinogram, non lineal model. 1. INTRODUCTION high concentrations. Double cones have L visual pigments and are screened by a variety of galloxanthin and -carotene A critical question in visual sciences is to determinate the types of photoreceptors that contribute - for a particular eye - mixtures [2, 8, 10-12]. The final cone mechanism sensitivity to the overall retinal spectral sensitivity. We have developed a is then determined by combining the cone visual pigment mathematical model that helps to answer this question. As a absorption and oil-droplet transmittance. In many birds, case study, we have used the electroretinogram results of a ultraviolet (UV) is a color that is believed to be involved in diurnal bird, the Firecrown hummingbirds. -

Colombia Mega II 1St – 30Th November 2016 (30 Days) Trip Report
Colombia Mega II 1st – 30th November 2016 (30 Days) Trip Report Black Manakin by Trevor Ellery Trip Report compiled by tour leader: Trevor Ellery Trip Report – RBL Colombia - Mega II 2016 2 ___________________________________________________________________________________ Top ten birds of the trip as voted for by the Participants: 1. Ocellated Tapaculo 6. Blue-and-yellow Macaw 2. Rainbow-bearded Thornbill 7. Red-ruffed Fruitcrow 3. Multicolored Tanager 8. Sungrebe 4. Fiery Topaz 9. Buffy Helmetcrest 5. Sword-billed Hummingbird 10. White-capped Dipper Tour Summary This was one again a fantastic trip across the length and breadth of the world’s birdiest nation. Highlights were many and included everything from the flashy Fiery Topazes and Guianan Cock-of- the-Rocks of the Mitu lowlands to the spectacular Rainbow-bearded Thornbills and Buffy Helmetcrests of the windswept highlands. In between, we visited just about every type of habitat that it is possible to bird in Colombia and shared many special moments: the diminutive Lanceolated Monklet that perched above us as we sheltered from the rain at the Piha Reserve, the showy Ochre-breasted Antpitta we stumbled across at an antswarm at Las Tangaras Reserve, the Ocellated Tapaculo (voted bird of the trip) that paraded in front of us at Rio Blanco, and the male Vermilion Cardinal, in all his crimson glory, that we enjoyed in the Guajira desert on the final morning of the trip. If you like seeing lots of birds, lots of specialities, lots of endemics and enjoy birding in some of the most stunning scenery on earth, then this trip is pretty unbeatable. -

Vogelliste Venezuela
Vogelliste Venezuela Datum: www.casa-vieja-merida.com (c) Beobachtungstage: 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Birdlist VENEZUELA copyrightBeobachtungsgebiete: Henri Pittier Azulita / Catatumbo La Altamira St Domingo Paramo Los Llanos Caura Sierra de Imataca Sierra de Lema + Gran Sabana Sucre Berge und Kueste Transfers Andere - gesehen gesehen an wieviel Tagen TINAMIFORMES: Tinamidae - Steißhühner 0 1 Tawny-breasted Tinamou Nothocercus julius Gelbbrusttinamu 0 2 Highland Tinamou Nothocercus bonapartei Bergtinamu 0 3 Gray Tinamou Tinamus tao Tao 0 4 Great Tinamou Tinamus major Großtinamu x 0 5 White-throated Tinamou Tinamus guttatus Weißkehltinamu 0 6 Cinereous Tinamou Crypturellus cinereus Grautinamu x x 0 7 Little Tinamou Crypturellus soui Brauntinamu x x x 0 8 Tepui Tinamou Crypturellus ptaritepui Tepuitinamu by 0 9 Brown Tinamou Crypturellus obsoletus Kastanientinamu 0 10 Undulated Tinamou Crypturellus undulatus Wellentinamu 0 11 Gray-legged Tinamou Crypturellus duidae Graufußtinamu 0 12 Red-legged Tinamou Crypturellus erythropus Rotfußtinamu birds-venezuela.dex x 0 13 Variegated Tinamou Crypturellus variegatus Rotbrusttinamu x x x 0 14 Barred Tinamou Crypturellus casiquiare Bindentinamu 0 ANSERIFORMES: Anatidae - Entenvögel 0 15 Horned Screamer Anhima cornuta Hornwehrvogel x 0 16 Northern Screamer Chauna chavaria Weißwangen-Wehrvogel x 0 17 White-faced Whistling-Duck Dendrocygna viduata Witwenpfeifgans x 0 18 Black-bellied Whistling-Duck Dendrocygna autumnalis Rotschnabel-Pfeifgans x 0 19 Fulvous Whistling-Duck Dendrocygna bicolor -

GCE Biology Question Paper Unit 02
For Examiner’s Use Centre Number Candidate Number Surname Other Names Examiner’s Initials Candidate Signature Question Mark General Certificate of Education 1 Advanced Subsidiary Examination June 2012 2 3 Biology BIOL2 4 Unit 2 The variety of living organisms 5 6 Monday 21 May 2012 1.30 pm to 3.15 pm 7 8 For this paper you must have: l a ruler with millimetre measurements. 9 l a calculator. TOTAL Time allowed l 1 hour 45 minutes Instructions l Use black ink or black ball-point pen. l Fill in the boxes at the top of this page. l Answer all questions. l You must answer the questions in the spaces provided. Do not write outside the box around each page or on blank pages. l You may ask for extra paper. Extra paper must be secured to this booklet. l Do all rough work in this book. Cross through any work you do not want to be marked. Information l The maximum mark for this paper is 85. l You are expected to use a calculator, where appropriate. l The marks for questions are shown in brackets. l Quality of Written Communication will be assessed in all answers. l You will be marked on your ability to: – use good English – organise information clearly – use scientific terminology accurately. (JUN12BIOL201) WMP/Jun12/BIOL2 BIOL2 Do not write 2 outside the box Answer all questions in the spaces provided. 1 (a) Flatworms are small animals that live in water. They have no specialised gas exchange or circulatory systems. The drawing shows one type of flatworm. -

Wild Hummingbirds Discriminate Nonspectral Colors
Wild hummingbirds discriminate nonspectral colors Mary Caswell Stoddarda,b,1, Harold N. Eysterb,c,2, Benedict G. Hogana,b,2, Dylan H. Morrisa, Edward R. Soucyd, and David W. Inouyeb,e aDepartment of Ecology and Evolutionary Biology, Princeton University, Princeton, NJ 08544; bRocky Mountain Biological Laboratory, Crested Butte, CO 81224; cInstitute for Resources, Environment and Sustainability, University of British Columbia, Vancouver, BC V6T 1Z4, Canada; dCenter for Brain Science, Harvard University, Cambridge, MA 02138; and eDepartment of Biology, University of Maryland, College Park, MD 20742 Edited by Scott V. Edwards, Harvard University, Cambridge, MA, and approved April 28, 2020 (received for review November 5, 2019) Many animals have the potential to discriminate nonspectral UV- or violet-sensitive (UVS/VS), short-wave–sensitive (SWS), colors. For humans, purple is the clearest example of a nonspectral medium-wave–sensitive (MWS), and long-wave–sensitive (LWS) color. It is perceived when two color cone types in the retina (blue color cones. Indirect evidence for avian tetrachromacy comes and red) with nonadjacent spectral sensitivity curves are pre- from the general agreement of behavioral data with a model that dominantly stimulated. Purple is considered nonspectral because predicts discrimination thresholds from opponent signals stem- no monochromatic light (such as from a rainbow) can evoke this ming from four single color cone types (8, 9). More directly, simultaneous stimulation. Except in primates and bees, few color-matching experiments (10) and tests designed to stimulate behavioral experiments have directly examined nonspectral color discrimination, and little is known about nonspectral color per- specific photoreceptors (11, 12) have suggested that avian color ception in animals with more than three types of color photore- vision results from at least three different opponent mechanisms ceptors. -

Pousada Rio Roosevelt: a Provisional Avifaunal Inventory in South
Cotinga31-090608:Cotinga 6/8/2009 2:38 PM Page 23 Cotinga 31 Pousada Rio Roosevelt: a provisional avifaunal inventory in south- western Amazonian Brazil, with information on life history, new distributional data and comments on taxonomy Andrew Whittaker Received 26 November 2007; final revision accepted 16 July 2008 first published online 4 March 2009 Cotinga 31 (2009): 23–46 Apresento uma lista preliminar de aves da Pousada Rio Roosevelt situada ao sul do rio Amazonas e leste do rio Madeira, do qual o Rio Roosevelt é um dos maiores afluentes da margem direta. A localização geográfica do pousada aumenta a importância da publicação de uma lista preliminar da avifauna, uma vez que ela se situa no interflúvio Madeira / Tapajós dentro do centro de endemismo Rondônia. Recentes descobertas ornitológicas neste centro de endemismo incluem a choca-de- garganta-preta Clytoctantes atrogularis, que foi encontrada na pousada e é considerada uma espécie globalmente ameaçada. Discuto porque a realização de levantamentos de aves na Amazônia é tão difícil, mencionando sucintamente alguns avanços ornitólogos Neotropicais principalmente com relação ao conhecimento das vocalizações das espécies. Os resultados obtidos confirmaram que o rio Roosevelt é uma importante barreira biográfica para algumas de Thamnophildae, família representada por 50 espécies na Pousada Roosevelt, localidade com a maior diversidade de espécies desta família em todo o mundo. Ao todo, um total de 481 espécies de aves foi registrado durante 51 dias no campo, indicando que estudos adicionais poderão elevar esse número para além de 550 espécies. Para cada espécie registrada são fornecidos detalhes sobre sua abundância, migração, preferências de hábitat e tipo de documentação na área. -

Visual Pigments and Oil Droplets from Six Classes of Photoreceptor in the Retinas of Birds J
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector Vision Res., Vol. 37, No. 16, pp. 2183-2194, 1997 Pergamon © 1997 Elsevier Science Ltd. All rights reserved PH: S0042-6989(97)00026-6 Printed in Great Britain 0042-6989/97 $17.00 + 0.00 Visual Pigments and Oil Droplets from Six Classes of Photoreceptor in the Retinas of Birds J. K. BOWMAKER,*:~ L. A. HEATH,~ S. E. WILKIE,-~ D. M. HUNT~" Received 8 August 1996; in revised form 2 December 1996 Microspectrophotometric examination of the retinal photoreceptors of the budgerigar (shell parakeet), Melopsittacus undulatus (Psittaciformes) and the zebra finch, Taeniopygia guttata (Passeriformes), demonstrate the presence of four, speetrally distinct classes of single cone that contain visual pigments absorbing maximally at about 565, 507, 430-445 and 360-380 nm. The three longer-wave cone classes contain coloured oil droplets acting as long pass filters with cut-offs at about 570, 500-520 and 445 nm, respectively, whereas the ultraviolet-sensitive cones contain a transparent droplet. The two species possess double cones in which both members contain the long- wave-sensitive visual pigment, but only the principal member contains an oil droplet, with cut-off at about 420 nm. A survey of the cones of the pigeon, Columba livia (Columbiformes), confirms the presence of the three longer-wave classes of single cone, but also reveals the presence of a fourth class containing a visual pigment with maximum absorbance at about 409 nm, combined with a transparent droplet. No evidence was found for a fifth, ultraviolet-sensitive receptor. -

Manaus, Brazil: Amazon Rainforest & River Islands
MANAUS, BRAZIL: AMAZON RAINFOREST & RIVER ISLANDS OCTOBER 4–17, 2019 What simply has to be one of the most beautiful hummingbirds, the Crimson Topaz — Photo: Andrew Whittaker LEADER: ANDREW WHITTAKER LIST COMPILED BY: ANDREW WHITTAKER VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM MANAUS, BRAZIL: AMAZON RAINFOREST & RIVER ISLANDS October 4–17, 2019 By Andrew Whittaker Manaus, without doubt, is one of the world’s major birding crossroads, located smack in the middle of the immense Amazon rainforest, 5,500,000 km 2 (2,123,562 sq mi), home to the richest and most mega diverse biome on our planet! This tour, as usual, offered a perfect opportunity to joyfully immerse ourselves into this fascinating birding and natural history bonanza. I have many fond memories of Manaus, as it was my home for more than 25 years and is always full of exciting surprises. I quickly learned that Amazonia never likes to give up any of its multitude of secrets easily, and, wow, there are so many still to discover! Immense rainforest canopy as far as the eye can see of the famous INPA tower — Photo: Andrew Whittaker Victor Emanuel Nature Tours 2 Manaus, Brazil, 2019 Amazonia is much more than just the rainforest, as we quickly learned. We also enjoyed exploring the mighty Amazon waterways on our relaxed boat trips, birding avian-rich river islands while being delighted by the exuberant rainforests on either side of the Negro, each with varied and unique species and different microhabitats. Amazonia never fails, and we certainly had our fair share of many delightful, stunning, and unique avian moments together. -

Distribution, Variation, and Taxonomy of Topaza Hummingbirds (Aves: Trochilidae)
ORNITOLOGIA NEOTROPICAL 11: 123–142, 2000 © The Neotropical Ornithological Society DISTRIBUTION, VARIATION, AND TAXONOMY OF TOPAZA HUMMINGBIRDS (AVES: TROCHILIDAE) Da-Shih Hu1, Leo Joseph2, & David Agro2 1Department of Psychiatry, Dartmouth Medical School, Hanover NH 03755, USA. 2Department of Ornithology, Academy of Natural Sciences, 1900 Benjamin Franklin Parkway, Philadelphia, PA 19103-1195, USA. Abstract. We review the distribution, variation, and taxonomy of the Crimson Topaz (Topaza pella) and the Fiery Topaz (Topaza pyra). Both taxa show sexual dimorphism of foot color and notable age-related varia- tion in plumage. Topaza pyra shows distinct and reliable differences from T. pella and warrants specific sta- tus. We describe a new subspecies, T. pyra amaruni, and delineate diagnostic features for T. pella pella, T. pella smaragdula, and T. pella microrhyncha. Topaza pella pamprepta is based on specimens with incorrect locality data and should be regarded as a synonym of T. p. smaragdula. We note recent range extensions for both species. Our findings serve, we hope, to illuminate directions for further research on these hummingbirds. Accepted 10 June 1999. Resumen. Revisamos la distribución, la variación, y la taxonomía del Topacio Carmesí (Topaza pella) y del Topacio Fuego (Topaza pyra). Los dos taxa demuestran dimorfismo sexual en el color del pie y notable variación en el plumaje con relación a la edad. Topaza pyra se distingue claramente de T. pella y merece la categoría de especie. Describimos una nueva subespecie, T. pyra amaruni, y presentamos los elementos dia- gnósticos para T. pella pella, T. pella smaragdula, y T. pella microrhyncha. Topaza pella pamprepta fue descrita en base a especímenes con localidades incorrectas y debe ser considerada como un sinónimo de T. -

Microspectrophotometry of Visual Pigments and Oil Droplets in A
The Journal of Experimental Biology 207, 1229-1240 1229 Published by The Company of Biologists 2004 doi:10.1242/jeb.00857 Microspectrophotometry of visual pigments and oil droplets in a marine bird, the wedge-tailed shearwater Puffinus pacificus: topographic variations in photoreceptor spectral characteristics Nathan S. Hart* Vision, Touch and Hearing Research Centre, School of Biomedical Sciences, University of Queensland, Brisbane, Queensland 4072, Australia *e-mail: [email protected] Accepted 5 January 2004 Summary Microspectrophotometric examination of the retina of a only the principal member contained an oil droplet, which procellariiform marine bird, the wedge-tailed shearwater had a λcut at 413·nm. The retina had a horizontal band or Puffinus pacificus, revealed the presence of five different ‘visual streak’ of increased photoreceptor density running types of vitamin A1-based visual pigment in seven across the retina approximately 1.5·mm dorsal to the top different types of photoreceptor. A single class of rod of the pecten. Cones in the centre of the horizontal streak contained a medium-wavelength sensitive visual pigment were smaller and had oil droplets that were either with a wavelength of maximum absorbance (λmax) at transparent/colourless or much less pigmented than at the 502·nm. Four different types of single cone contained periphery. It is proposed that the reduction in cone oil visual pigments maximally sensitive in either the violet droplet pigmentation in retinal areas associated with (VS, λmax 406·nm), short (SWS, λmax 450·nm), medium high visual acuity is an adaptation to compensate for (MWS, λmax 503·nm) or long (LWS, λmax 566·nm) spectral the reduced photon capture ability of the narrower ranges. -

Classification, Evolution & Biodiversity Class Questions
WJEC Classification, Evolution & Biodiversity Class Questions Tyrone. R.L. John 1 Tyrone. R.L. John 2 Tyrone. R.L. John 3 6 2 When a new species is discovered, it needs to be classified. (a) Define the term classification. ................................................................................................................................................... ................................................................................................................................................... ................................................................................................................................................... ................................................................................................................................................... .............................................................................................................................................. [2] (b) (i) Suggest what criteria a taxonomist may take into account when classifying a new species. ........................................................................................................................................... ........................................................................................................................................... ........................................................................................................................................... ..........................................................................................................................................