Degradation of Natural and Synthetic Fibers in Various Aqueous Environments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
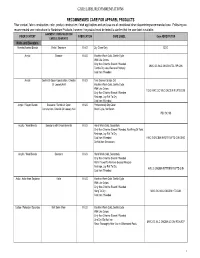
Care Label Recommendations
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Choosing the Proper Short Cut Fiber for Your Nonwoven Web
Choosing The Proper Short Cut Fiber for Your Nonwoven Web ABSTRACT You have decided that your web needs a synthetic fiber. There are three important factors that have to be considered: generic type, diameter, and length. In order to make the right choice, it is important to know the chemical and physical characteristics of the numerous man-made fibers, and to understand what is meant by terms such as denier and denier per filament (dpf). PROPERTIES Denier Denier is a property that varies depending on the fiber type. It is defined as the weight in grams of 9,000 meters of fiber. The current standard of denier is 0.05 grams per 450 meters. Yarn is usually made up of numerous filaments. The denier of the yarn divided by its number of filaments is the denier per filament (dpf). Thus, denier per filament is a method of expressing the diameter of a fiber. Obviously, the smaller the denier per filament, the more filaments there are in the yarn. If a fairly closed, tight web is desired, then lower dpf fibers (1.5 or 3.0) are preferred. On the other hand, if high porosity is desired in the web, a larger dpf fiber - perhaps 6.0 or 12.0 - should be chosen. Here are the formulas for converting denier into microns, mils, or decitex: Diameter in microns = 11.89 x (denier / density in grams per milliliter)½ Diameter in mils = diameter in microns x .03937 Decitex = denier x 1.1 The following chart may be helpful. Our stock fibers are listed along with their density and the diameter in denier, micron, mils, and decitex for each: Diameter Generic Type -

Silk, Linen, Leather, Denim, Grass, Cotton, Felt
Silk, linen, leather, denim, grass, cotton, felt. Natural materials keep the integrity of their shape yet hold an impression of the figure that has worn them. Fit-out for Olivia Spencer Bower speaks to the fabrics and forms that we live in. An assortment of garment patterns, building plans and the lifestyles of art heroines provide templates or underlying structures for Emma Fitts’ installation at the Ilam School of Fine Arts gallery. Adopting the layout of a modernist home and featuring a series of fabric hangings and clothing cutouts, Fitts’ work acutely relates to our situated knowledge, the proportions of the body and our experience of space, while also implying much less measurable qualities. Alternative histories that reveal the social relationships and values of a bygone era are incorporated to form a homage of sorts, though these references are evoked to shed light on the present–what it means to live and make work as an artist today. The gallery is physically divided by four large textile works, hung from the ceiling according to the walls shown in architectural plans for 15A Leinster Avenue, Christchurch–the former home of artist Olivia Spencer Bower (1905- 82). These soft walls enable visitors to occupy the space as though wandering through her actual house: living room, kitchen, sunroom, bedroom and studio. Designed in 1969 by architects Cowey and McGregor in the Christchurch style of neo-brutalism, the house was commissioned to accommodate the needs of a female artist living alone. There is an emphasis on form developed in relation to function and it is both refined and compact, manifesting these ideals. -

“Al-Tally” Ascension Journey from an Egyptian Folk Art to International Fashion Trend
مجمة العمارة والفنون العدد العاشر “Al-tally” ascension journey from an Egyptian folk art to international fashion trend Dr. Noha Fawzy Abdel Wahab Lecturer at fashion department -The Higher Institute of Applied Arts Introduction: Tally is a netting fabric embroidered with metal. The embroidery is done by threading wide needles with flat strips of metal about 1/8” wide. The metal may be nickel silver, copper or brass. The netting is made of cotton or linen. The fabric is also called tulle-bi-telli. The patterns formed by this metal embroidery include geometric figures as well as plants, birds, people and camels. Tally has been made in the Asyut region of Upper Egypt since the late 19th century, although the concept of metal embroidery dates to ancient Egypt, as well as other areas of the Middle East, Asia, India and Europe. A very sheer fabric is shown in Ancient Egyptian tomb paintings. The fabric was first imported to the U.S. for the 1893 Chicago. The geometric motifs were well suited to the Art Deco style of the time. Tally is generally black, white or ecru. It is found most often in the form of a shawl, but also seen in small squares, large pieces used as bed canopies and even traditional Egyptian dresses. Tally shawls were made into garments by purchasers, particularly during the 1920s. ملخص البحث: التمي ىو نوع من انواع االتطريز عمى اقمشة منسوجة ويتم ىذا النوع من التطريز عن طريق لضم ابر عريضة بخيوط معدنية مسطحة بسمك 1/8" تصنع ىذه الخيوط من النيكل او الفضة او النحاس.واﻻقمشة المستخدمة في صناعة التمي تكون مصنوعة اما من القطن او الكتان. -

Analysis of the Polyester Clothing Value Chain to Identify Key Intervention Points for Sustainability Cristina Palacios‑Mateo* , Yvonne Van Der Meer and Gunnar Seide
Palacios‑Mateo et al. Environ Sci Eur (2021) 33:2 https://doi.org/10.1186/s12302‑020‑00447‑x REVIEW Open Access Analysis of the polyester clothing value chain to identify key intervention points for sustainability Cristina Palacios‑Mateo* , Yvonne van der Meer and Gunnar Seide Abstract Clothing is one of the primary human needs, and the demand is met by the global production of thousands of tons of textile fbers, fabrics and garments every day. Polyester clothing manufactured from oil‑based polyethylene tereph‑ thalate (PET) is the market leader. Conventional PET creates pollution along its entire value chain—during the produc‑ tion, use and end‑of‑life phases—and also contributes to the unsustainable depletion of resources. The consumption of PET garments thus compromises the quality of land, water and air, destroys ecosystems, and endangers human health. In this article, we discuss the diferent stages of the value chain for polyester clothing from the perspective of sustainability, describing current environmental challenges such as pollution from textile factory wastewater, and microfbers released from clothing during the laundry cycle. We also consider potential solutions such as enhanced reuse and recycling. Finally, we propose a series of recommendations that should be applied to polyester clothing at all stages along the value chain, ofering the potential for meaningful and efective change to improve the environ‑ mental sustainability of polyester textiles on a global scale. Keywords: PET, Textiles, Value chain, Environmental sustainability, Microfbers, Pollution, Recycling, Life cycle Introduction with 2019 estimates in Germany suggesting an average Te global volume of fber production for textile manu- lifetime of only 4.4 years [5]. -

Performance Evaluation of 3-D Basalt Fiber Reinforced Concrete & Basalt
Highway IDEA Program Performance Evaluation of 3-D Basalt Fiber Reinforced Concrete & Basalt Rod Reinforced Concrete Final Report for Highway IDEA Project 45 Prepared by: V Ramakrishnan and Neeraj S. Tolmare, South Dakota School of Mines & Technology, Rapid City, SD Vladimir B. Brik, Research & Technology, Inc., Madison, WI November 1998 IDEA PROGRAM F'INAL REPORT Contract No. NCHRP-45 IDEA Program Transportation Resea¡ch Board National Research Council November 1998 PERFORMANCE EVALUATION OF 3.I) BASALT ITIBER REINFORCED CONCRETE & BASALT R:D RETNFORCED CONCRETE V. Rarnalaishnan Neeraj S. Tolmare South Dakota School of Mines & Technolory, Rapid City, SD Principal Investigator: Madimir B. Brik Research & Technology, lnc., Madison, WI INNOVATIONS DESERVING EXPLORATORY ANALYSIS (IDEA) PROGRAMS MANAGED BY THE TRANSPORTATION RESEARCH BOARD (TRB) This NCHRP-IDEA investigation was completed as part of the National Cooperative Highway Research Program (NCHRP). The NCHRP-IDEA program is one of the four IDEA programs managed by the Transportation Research Board (TRB) to foster innovations in highway and intermodal surface transportation systems. The other three IDEA program areas are Transit-IDEA, which focuses on products and results for transit practice, in support of the Transit Cooperative Research Program (TCRP), Safety-IDEA, which focuses on motor carrier safety practice, in support of the Federal Motor Carrier Safety Administration and Federal Railroad Administration, and High Speed Rail-IDEA (HSR), which focuses on products and results for high speed rail practice, in support of the Federal Railroad Administration. The four IDEA program areas are integrated to promote the development and testing of nontraditional and innovative concepts, methods, and technologies for surface transportation systems. -

Sea-Silk Based Nanofibers and Their Diameter Prediction THERMAL SCIENCE: Year 2019, Vol
Tian, D., et al.: Sea-Silk Based Nanofibers and Their Diameter Prediction THERMAL SCIENCE: Year 2019, Vol. 23, No. 4, pp. 2253-2256 2253 SEA-SILK BASED NANOFIBERS AND THEIR DIAMETER PREDICTION by * Dan TIAN, Chan-Juan ZHOU, and Ji-Huan HE National Engineering Laboratory for Modern Silk, College of Textile and Clothing Engineering, Soochow University, Suzhou, China Original scientific paper https://doi.org/10.2298/TSCI1904253T Diameter of sea-silk based nanofibers prepared by electrospinning is closely re- lated to the concentrations of sea-silk solution. A mathematical model is estab- lished according the mass conservation law in fluid mechanics to predict the di- ameter of fibers, and the MATLAB software is used to fit the experiment value. The results show that the fitted equation is quite accurate and efficient for estimating the diameter of fibers with different concentrations. Key words: electrospinning, sea-silk, mathematical model, nanofiber, fiber’s diameter. Introduction Electrospinning is an effective way to prepare nanofibers, it is a fabrication process that uses an electric field to control the deposition of polymer fibers onto a receptor [1-8]. In electrospinning process, the diameter of nanofiber is determined by many factors, like volt- age, viscosity of solution, receptor’s distance, environment temperature, environment humidi- ty, etc. [9, 10]. Nanofiber’s diameter and morphology can also be controlled by additives [11]. The sea silk is one of the oldest natural silks, which has a history of more than 5000 years [12-16], and now we can produce artificial sea silk through Mytilus edulis. To this end, we extract protein from Mytilus edulis and then use it as an additive for electrospinning, this maybe has some effects on the morphology of fibers. -

Silk Cotton Vs. Bombax Vs. Banyan
Ceiba pentandra Kopok tree, Silk-cotton tree Ta Prohm, Cambodia By Isabel Zucker Largest known specimen in Lal Bagh Gardens in Bangalore, India. http://scienceray.com/biology/botany/amazing-trees-from-around-the-world-the-seven-wonder-trees/ Ceiba pentandra Taxonomy • Family: Malvaceae • Sub family: Bombacaceae -Bombax spp. in same family - much online confusion as to which tree is primarily in Ta Praham, Cambodia. • Fig(Moraceae), banyan and kapok trees in Ta Praham • Often referred to as a banyan tree, which is quite confusing. Distribution • Originated in the American tropics, natural and human distribution. • Africa, Asia. – Especially Indonesia and Thailand • Indian ocean islands • Ornamental shade tree • Zone – Humid areas, rainforest, dry areas – Mean annual precipitation 60-224 inches per year – Temperatures ranging from 73-80 unaffected by frost – Elevation from 0-4,500 feet – Dry season ranging from 0-6 months Characteristics • Rapidly growing, deciduous • Reaches height up to 200 feet • Can grow 13 feet per year • Diameter up to 9 feet above buttress – Buttress can extend 10 feet from the trunk and be 10 feet tall • large umbrella-shaped canopies emerge above the forest canopy • http://www.flmnh.ufl.edu/caribarch/ceiba.htm • Home to many animals – Birds, frogs, insects – Flowers open in the evening, pollinated by bats • Epiphytes grow in branches • Compound leaves with 5-8 lance- shaped leaflets 3-8 inches long • Dense clusters of whitish to pink flowers December to February – 3-6 inch long, elliptical fruits. – Seeds of fruit surrounded by dense, cottony fibers. – Fibers almost pure cellulose, buoyant, impervious to water, low thermal conductivity, cannot be spun. -

DID YOU KNOW? PET (Polyethylene Terephthalate) Is Actually Polyester. When PET Is Used for Bottles, Containers and Other
355 Lexington Ave., Suite 1500 ▪ New York, NY 10017 ▪ www.PETresin.org DID YOU KNOW? Little-Known Facts about PET Plastic . PET (polyethylene terephthalate) is actually polyester. When PET is used for bottles, containers and other applications, it is called PET or PET resin. When PET is used as a fiber, it is typically called polyester. The PET bottle was invented by Nathaniel C. Wyeth, a DuPont engineer and brother of American painter Andrew Wyeth. The patent was issued to Wyeth in 1973 and assigned to DuPont. According to the EPA, recycling one pound of PET bottles (that’s about 10 two-liter soda bottles) saves approximately 26,000 BTUs of energy. PET bottles and the sun are helping millions of people in developing countries obtain potable water. Using a system called SODIS (solar water disinfection), inhabitants set water-filled PET bottles in the sun for several hours or days – depending on how much sunlight is available – as a simple but effective means of destroying disease-causing bacteria and gaining safe drinking water. More than 1.5 billion pounds of used PET bottles and containers are collected in the U.S. each year for recycling. PET is the most recycled plastic in the U.S. and the world. A single-serve PET bottle (0.5 liter) is strong enough to hold 50 times its weight in water. Chemists keep finding new ways to make PET lighter without losing any strength. A 2-liter PET bottle that weighed 68 grams in 1980 now weighs as little as 42 grams. The average weight of single-serve 0.5 liter PET water bottle is now 9.9 grams, nearly half of what it weighed in 2000. -

Handle and Comfort Characteristics of Cotton Core Spun Lycra and Polyester/ Lycra Fabrics for Application As Blouse Nirmala Varghese and G
Volume 8, Issue 4, Spring2014 Handle and Comfort Characteristics of Cotton Core Spun Lycra and Polyester/ Lycra Fabrics for Application as Blouse Nirmala Varghese and G. Thilagavathi Department of Apparel & Fashion Technology PSG College of Technology Tamil Nadu, India ABSTRACT Stretchable plain woven fabrics are developed with 60s cotton core spun Lycra and polyester Lycra twisted yarn (91D) in weft with cotton, silk and polyester in warp. The influence of fabric specifications on thermal comfort, low stress mechanical properties, primary and total hand value is analyzed. The results indicated that cotton core spun Lycra woven with polyester warp provided higher value of thermal conductivity, air permeability and water vapor permeability. The woven stretch fabrics produced with polyester warp, cotton core spun Lycra and polyester Lycra in weft has excellent aesthetic and drape properties. These fabrics have higher tensile resilience, tensile strain, lower shear rigidity, superior primary hand and a higher total hand value of 4.08 and 3.93 respectively and are best suitable for women’s blouse. Keywords: Cotton core spun Lycra, polyester/Lycra, woven stretch, fabric handle, sari blouse Introduction Lycra and polyester/lycra twisted yarn. Several studies have been carried out Stretch is often the most important investigating the physical properties of core property of textiles that contribute to wearer spun yarn, cotton/lycra and properties of the comfort. The stretch yarn and fabrics are fabrics containing different rates of spandex widely used and preferred by the people in yarns. Core spun cotton/spandex shows high the last few years all over the world because resiliency property than 100% cotton yarns, of their stretch ability and shape retention due to its soft and rubbery isocyanate properties. -

New Hemp-Based Fiber Enhances Wet-Mix Shotcrete Performance
New Hemp-Based Fiber Enhances Wet-Mix Shotcrete Performance By Dudley R. (Rusty) Morgan, Lihe (John) Zhang, and Mike Pildysh n innovative processed natural hemp-based fiber without any fibers and mixtures with a synthetic (polypro- has been developed for use in concrete and shot- pylene) microfiber. These studies, conducted in Vancouver, A crete (wet- and dry-mix shotcrete processes) in lieu BC, Canada, demonstrated that when used at equivalent of conventional synthetic fibers, which are derived from fiber-volume addition rates, not only was the natural hemp- hydro-carbon (oil and/or gas) feedstock. A driving force based fiber more effective in mitigating plastic shrinkage behind this development has been the desire to produce cracking than synthetic microfiber but it also provided many a truly sustainable green fiber with dramatic reductions in additional benefits in the plastic and hardened concretes. This was particularly true for the wet-mix shotcrete process, CO2 emissions into the atmosphere during fiber production, compared to the synthetic (mainly polypropylene) fibers cur- where some marked benefits to the shotcrete application rently being used in the concrete and shotcrete industries. and finishing processes were found. Synthetic microfibers have mainly been used in concrete This article provides a summary of the findings from and shotcrete to help mitigate early-age plastic shrinkage the wet-mix shotcrete laboratory study. It also provides cracking. In the absence of adequate protection and initial observations from a subsequent full-scale field application curing, plastic shrinkage cracking has been an issue in the of wet-mix shotcrete with the natural hemp-based fiber, shotcrete industry. -
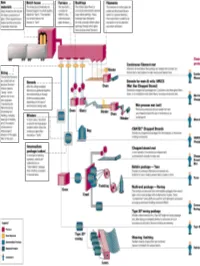
All About Fibers
RawRaw MaterialsMaterials ¾ More than half the mix is silica sand, the basic building block of any glass. ¾ Other ingredients are borates and trace amounts of specialty chemicals. Return © 2003, P. Joyce BatchBatch HouseHouse && FurnaceFurnace ¾ The materials are blended together in a bulk quantity, called the "batch." ¾ The blended mix is then fed into the furnace or "tank." ¾ The temperature is so high that the sand and other ingredients dissolve into molten glass. Return © 2003, P. Joyce BushingsBushings ¾The molten glass flows to numerous high heat-resistant platinum trays which have thousands of small, precisely drilled tubular openings, called "bushings." Return © 2003, P. Joyce FilamentsFilaments ¾This thin stream of molten glass is pulled and attenuated (drawn down) to a precise diameter, then quenched or cooled by air and water to fix this diameter and create a filament. Return © 2003, P. Joyce SizingSizing ¾The hair-like filaments are coated with an aqueous chemical mixture called a "sizing," which serves two main purposes: 1) protecting the filaments from each other during processing and handling, and 2) ensuring good adhesion of the glass fiber to the resin. Return © 2003, P. Joyce WindersWinders ¾ In most cases, the strand is wound onto high-speed winders which collect the continuous fiber glass into balls or "doffs.“ Single end roving ¾ Most of these packages are shipped directly to customers for such processes as pultrusion and filament winding. ¾ Doffs are heated in an oven to dry the chemical sizing. Return © 2003, P. Joyce IntermediateIntermediate PackagePackage ¾ In one type of winding operation, strands are collected into an "intermediate" package that is further processed in one of several ways.