Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug Class Review Beta Adrenergic Blockers
Drug Class Review Beta Adrenergic Blockers Final Report Update 4 July 2009 Update 3: September 2007 Update 2: May 2005 Update 1: September 2004 Original Report: September 2003 The literature on this topic is scanned periodically. The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use, or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Mark Helfand, MD, MPH Kim Peterson, MS Vivian Christensen, PhD Tracy Dana, MLS Sujata Thakurta, MPA:HA Drug Effectiveness Review Project Marian McDonagh, PharmD, Principal Investigator Oregon Evidence-based Practice Center Mark Helfand, MD, MPH, Director Oregon Health & Science University Copyright © 2009 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Report Update 4 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION .......................................................................................................................... 6 Purpose and Limitations of Evidence Reports........................................................................................ 8 Scope and Key Questions .................................................................................................................... 10 METHODS................................................................................................................................. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Drugs for Primary Prevention of Atherosclerotic Cardiovascular Disease: an Overview of Systematic Reviews
Supplementary Online Content Karmali KN, Lloyd-Jones DM, Berendsen MA, et al. Drugs for primary prevention of atherosclerotic cardiovascular disease: an overview of systematic reviews. JAMA Cardiol. Published online April 27, 2016. doi:10.1001/jamacardio.2016.0218. eAppendix 1. Search Documentation Details eAppendix 2. Background, Methods, and Results of Systematic Review of Combination Drug Therapy to Evaluate for Potential Interaction of Effects eAppendix 3. PRISMA Flow Charts for Each Drug Class and Detailed Systematic Review Characteristics and Summary of Included Systematic Reviews and Meta-analyses eAppendix 4. List of Excluded Studies and Reasons for Exclusion This supplementary material has been provided by the authors to give readers additional information about their work. © 2016 American Medical Association. All rights reserved. 1 Downloaded From: https://jamanetwork.com/ on 09/28/2021 eAppendix 1. Search Documentation Details. Database Organizing body Purpose Pros Cons Cochrane Cochrane Library in Database of all available -Curated by the Cochrane -Content is limited to Database of the United Kingdom systematic reviews and Collaboration reviews completed Systematic (UK) protocols published by by the Cochrane Reviews the Cochrane -Only systematic reviews Collaboration Collaboration and systematic review protocols Database of National Health Collection of structured -Curated by Centre for -Only provides Abstracts of Services (NHS) abstracts and Reviews and Dissemination structured abstracts Reviews of Centre for Reviews bibliographic -

(12) United States Patent (10) Patent No.: US 8,026,285 B2 Bezwada (45) Date of Patent: Sep
US008O26285B2 (12) United States Patent (10) Patent No.: US 8,026,285 B2 BeZWada (45) Date of Patent: Sep. 27, 2011 (54) CONTROL RELEASE OF BIOLOGICALLY 6,955,827 B2 10/2005 Barabolak ACTIVE COMPOUNDS FROM 2002/0028229 A1 3/2002 Lezdey 2002fO169275 A1 11/2002 Matsuda MULT-ARMED OLGOMERS 2003/O158598 A1 8, 2003 Ashton et al. 2003/0216307 A1 11/2003 Kohn (75) Inventor: Rao S. Bezwada, Hillsborough, NJ (US) 2003/0232091 A1 12/2003 Shefer 2004/0096476 A1 5, 2004 Uhrich (73) Assignee: Bezwada Biomedical, LLC, 2004/01 17007 A1 6/2004 Whitbourne 2004/O185250 A1 9, 2004 John Hillsborough, NJ (US) 2005/0048121 A1 3, 2005 East 2005/OO74493 A1 4/2005 Mehta (*) Notice: Subject to any disclaimer, the term of this 2005/OO953OO A1 5/2005 Wynn patent is extended or adjusted under 35 2005, 0112171 A1 5/2005 Tang U.S.C. 154(b) by 423 days. 2005/O152958 A1 7/2005 Cordes 2005/0238689 A1 10/2005 Carpenter 2006, OO13851 A1 1/2006 Giroux (21) Appl. No.: 12/203,761 2006/0091034 A1 5, 2006 Scalzo 2006/0172983 A1 8, 2006 Bezwada (22) Filed: Sep. 3, 2008 2006,0188547 A1 8, 2006 Bezwada 2007,025 1831 A1 11/2007 Kaczur (65) Prior Publication Data FOREIGN PATENT DOCUMENTS US 2009/0076174 A1 Mar. 19, 2009 EP OO99.177 1, 1984 EP 146.0089 9, 2004 Related U.S. Application Data WO WO9638528 12/1996 WO WO 2004/008101 1, 2004 (60) Provisional application No. 60/969,787, filed on Sep. WO WO 2006/052790 5, 2006 4, 2007. -

Molecular Pharmacology of K Potassium Channels
Cellular Physiology Cell Physiol Biochem 2021;55(S3):87-107 DOI: 10.33594/00000033910.33594/000000339 © 2021 The Author(s).© 2021 Published The Author(s) by and Biochemistry Published online: online: 6 6March March 2021 2021 Cell Physiol BiochemPublished Press GmbH&Co. by Cell Physiol KG Biochem 87 Press GmbH&Co. KG, Duesseldorf Decher et al.: Molecular Pharmacology of K Channels Accepted: 12 January 2021 2P www.cellphysiolbiochem.com This article is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 Interna- tional License (CC BY-NC-ND). Usage and distribution for commercial purposes as well as any distribution of modified material requires written permission. Review Molecular Pharmacology of K2P Potassium Channels Niels Dechera Susanne Rinnéa Mauricio Bedoyab,c Wendy Gonzalezb,c Aytug K. Kipera aVegetative Physiology, Institute for Physiology and Pathophysiology, Philipps-University Marburg, Marburg, Germany, bCentro de Bioinformática y Simulación Molecular, Universidad de Talca, Talca, Chile, cMillennium Nucleus of Ion Channels-Associated Diseases (MiNICAD), Universidad de Talca, Talca, Chile Key Words Drug binding sites • K2P potassium channels • Ion channels • Molecular pharmacology Abstract Potassium channels of the tandem of two-pore-domain (K2P) family were among the last potassium channels cloned. However, recent progress in understanding their physiological relevance and molecular pharmacology revealed their therapeutic potential and thus these channels evolved as major drug targets against a large variety of diseases. However, after the initial cloning of the fifteen family members there was a lack of potent and/or selective modulators. By now a large variety of K2P channel modulators (activators and blockers) have been described, especially for TASK-1, TASK-3, TREK-1, TREK2, TRAAK and TRESK channels. -

A Unitary Mechanism of Calcium Antagonist Drug Action '(Dihydropyridine/Nifedipine/Verapamil/Neuroleptic/Diltiazem) KENNETH M
Proc. Nati Acad. Sci. USA Vol. 80, pp. 860-864, February 1983 Medical Sciences A unitary mechanism of calcium antagonist drug action '(dihydropyridine/nifedipine/verapamil/neuroleptic/diltiazem) KENNETH M. M. MURPHY, ROBERT J. GOULD, BRIAN L. LARGENT, AND SOLOMON H. SNYDER* Departments of Neuroscience, Pharmacology and Experimental Therapeutics, and Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 72S North Wolfe Street, Baltimore, Maryland 21205 Contributed by Solomon H. Snyder, October 21, 1982 ABSTRACT [3H]Nitrendipine binding to drug receptor sites liquid scintillation counting were carried out as described (7). associated with calcium channels is allosterically regulated by a All experiments, performed in triplicate, were replicated at diverse group of calcium channel antagonists. Verapamil, D-600 least three times with similar results. (methoxyverapamit), tiapamil, lidoflazine, flunarizine, cinnari- Guinea pig ileum longitudinal muscles were prepared for zine, and prenylamine all reduce P3H]nitrendipine binding affin- recording as described by Rosenberger et aL (13) and incubated ity. By contrast, diltiazem, a benzothiazepine calcium channel an- in a modified Tyrode's buffer (14) at 370C with continuous aer- tagonist, enhances [3H]nitrendipine binding. All these drugeffects ation with 95% '02/5% CO2. Ileum longitudinal muscles were involve a single site allosterically linked to the [3H]nitrendipine incubated in this buffer for 30 min before Ca2"-dependent con- binding site. Inhibition of t3H]nitrendipine binding by prenyl- were as described Jim et aL amine, lidoflazine, or tiapamil is reversed by D-600and diltiazem, tractions recorded by (15). which alone respectively slightlyreduceorenhance H]mnitrendipine RESULTS binding. Diltiazem reverses the inhibition of [3H]nitrendipine binding by D-600. -

2000 Dialysis of Drugs
2000 Dialysis of Drugs PROVIDED AS AN EDUCATIONAL SERVICE BY AMGEN INC. I 2000 DIAL Dialysis of Drugs YSIS OF DRUGS Curtis A. Johnson, PharmD Member, Board of Directors Nephrology Pharmacy Associates Ann Arbor, Michigan and Professor of Pharmacy and Medicine University of Wisconsin-Madison Madison, Wisconsin William D. Simmons, RPh Senior Clinical Pharmacist Department of Pharmacy University of Wisconsin Hospital and Clinics Madison, Wisconsin SEE DISCLAIMER REGARDING USE OF THIS POCKET BOOK DISCLAIMER—These Dialysis of Drugs guidelines are offered as a general summary of information for pharmacists and other medical professionals. Inappropriate administration of drugs may involve serious medical risks to the patient which can only be identified by medical professionals. Depending on the circumstances, the risks can be serious and can include severe injury, including death. These guidelines cannot identify medical risks specific to an individual patient or recommend patient treatment. These guidelines are not to be used as a substitute for professional training. The absence of typographical errors is not guaranteed. Use of these guidelines indicates acknowledgement that neither Nephrology Pharmacy Associates, Inc. nor Amgen Inc. will be responsible for any loss or injury, including death, sustained in connection with or as a result of the use of these guidelines. Readers should consult the complete information available in the package insert for each agent indicated before prescribing medications. Guides such as this one can only draw from information available as of the date of publication. Neither Nephrology Pharmacy Associates, Inc. nor Amgen Inc. is under any obligation to update information contained herein. Future medical advances or product information may affect or change the information provided. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -
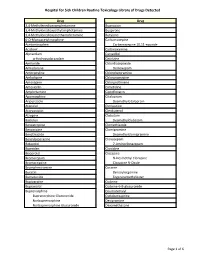
Page 1 S C Routine Toxicology Library Drug 3,4
Hospital for Sick Children Routine Toxicology Library of Drugs Detected Drug Drug 3,4-Methylenedioxyamphetamine Bupropion 3,4-Methylenedioxyethylamphetamine Buspirone 3,4-Methylenedioxymethamphetamine Butylone 6-O-Monoacetylmorphine Carbamazepine Acetaminophen Carbamazepine 10,11-epoxide Aciclovir Carbinoxamine Alprazolam Carvedilol α-Hydroxyalprazolam Cetirizine Amiloride Chlordiazepoxide Amiodarone Demoxepam Amitriptyline Chlorpheniramine Amlodipine Chlorpromazine Amoxapine Chlorprothixene Amoxicillin Cimetidine Amphetamine Ciprofloxacin Apomorphine Citalopram Aripiprazole Desmethylcitalopram Atenolol Clemastine Atorvastatin Clenbuterol Atropine Clobazam Baclofen Desmethylclobazam Benzatropine Clomethiazole Benzocaine Clomipramine Benzthiazide Desmethylclomipramine Benzylpiperazine Clonazepam Betaxolol 7-Aminoclonazepam Biperiden Clonidine Bisoprolol Clozapine Bromazepam N-Desmethyl Clozapine Bromocriptine Clozapine N-Oxide Brompheniramine Cocaine Bucetin Benzoylecgonine Bumetanide Ecgoninemethylester Bupivacaine Codeine Bupranolol Codeine-6-B-glucuronide Buprenorphine Coumatetralyl Buprenorphine Glucuronide Cyclobenzaprine Norbuprenorphine Desipramine Norbuprenorphine Glucuronide Dexamethasone Page 1 of 6 Hospital for Sick Children Routine Toxicology Library of Drugs Detected Drug Drug Dextromethorphan Fluoxetine Dextrorphan Norfluoxetine Diazepam Fluphenazine Nordiazepam Flurazepam 4-Hydroxynordiazepam Desalkylflurazepam Diclofenac 2-Hydroxyethylflurazepam Dihydrocodeine Fluvoxamine Dihydrocodeine-6-β-glucuronide Gabapentin Dihydroergotamine -

Pharmaceuticals (Monocomponent Products) ………………………..………… 31 Pharmaceuticals (Combination and Group Products) ………………….……
DESA The Department of Economic and Social Affairs of the United Nations Secretariat is a vital interface between global and policies in the economic, social and environmental spheres and national action. The Department works in three main interlinked areas: (i) it compiles, generates and analyses a wide range of economic, social and environmental data and information on which States Members of the United Nations draw to review common problems and to take stock of policy options; (ii) it facilitates the negotiations of Member States in many intergovernmental bodies on joint courses of action to address ongoing or emerging global challenges; and (iii) it advises interested Governments on the ways and means of translating policy frameworks developed in United Nations conferences and summits into programmes at the country level and, through technical assistance, helps build national capacities. Note Symbols of United Nations documents are composed of the capital letters combined with figures. Mention of such a symbol indicates a reference to a United Nations document. Applications for the right to reproduce this work or parts thereof are welcomed and should be sent to the Secretary, United Nations Publications Board, United Nations Headquarters, New York, NY 10017, United States of America. Governments and governmental institutions may reproduce this work or parts thereof without permission, but are requested to inform the United Nations of such reproduction. UNITED NATIONS PUBLICATION Copyright @ United Nations, 2005 All rights reserved TABLE OF CONTENTS Introduction …………………………………………………………..……..……..….. 4 Alphabetical Listing of products ……..………………………………..….….…..….... 8 Classified Listing of products ………………………………………………………… 20 List of codes for countries, territories and areas ………………………...…….……… 30 PART I. REGULATORY INFORMATION Pharmaceuticals (monocomponent products) ………………………..………… 31 Pharmaceuticals (combination and group products) ………………….……........ -

Classification of Antiarrhythmic Drugs Keitaro Hashimoto1,* 1Professor Emeritus, University of Yamanashi, 1110 Shimokato, Chuo, Yamanashi 409-3898, Japan
J Pharmacol Sci 103, 333 – 346 (2007) Journal of Pharmacological Sciences ©2007 The Japanese Pharmacological Society Critical Review Arrhythmia Models for Drug Research: Classification of Antiarrhythmic Drugs Keitaro Hashimoto1,* 1Professor Emeritus, University of Yamanashi, 1110 Shimokato, Chuo, Yamanashi 409-3898, Japan Received December 31, 2006 Abstract. The aim of this study was to classify antiarrhythmic drugs based on their effective- ness on 6 in vivo arrhythmia models, mainly using dogs. The models were produced by two-stage coronary ligation, digitalis, halothane-adrenaline, programmed electrical stimulation in old myo- cardial infarction dogs, coronary artery occlusion/reperfusion, or chronic atrioventricular block. Na+-channel–blocking drugs suppressed two-stage coronary ligation and digitalis arrhythmias. Ca2+-channel blockers and β-blockers suppressed halothane-adrenaline arrhythmia. Positive inotropic drugs aggravated halothane-adrenaline arrhythmia, but did not aggravate digitalis arrhythmia. K+-channel blockers suppressed programmed electrical stimulation induced arrhythmia, but induced torsades de pointes type arrhythmia in chronic atrioventricular block dogs and aggravated halothane-adrenaline arrhythmia. Na+/H+-exchange blockers suppressed coronary artery occlusion/reperfusion arrhythmias. This classification may be useful for predict- ing the clinical effectiveness in the preclinical stage of drug development. Keywords: arrhythmia model, antiarrhythmic drug, proarrhythmic potential of drugs, ion channel, classification -

Astra Pharma~Euticals Pty Ltd Acn Oog Ss2
Astra Pharma~euticals Pty Ltd A.c.N. oog ss2 311 M£y, 1992 Our reference Your letter Your reference To: All Hospital Pharmacies Attention: Chief Pharmacist Dear Sir/Madam, Availability of IMDUR™ Astra Pharmaceuticals Pty Ltd is pleased to announce the availability in Australia of new Imdur, the first once daily1 oral nitrate for prophylaxis of angina pectoris. Imdur is a new forni of nitrate, isosorbide mononitrate, in a sustained release formulation known as Durules™. The Durules sustained release l3ystem. is a product of ori15!naf'.AE!tra research. ~·~ ~ Imdur is available as a -6Qmg/scored_,til'blet in a memory pack of 30 and it was ;;j< launched on May !1-th 19~2-tO'Spectalists and General Practitioners. Imdur will ·be a"VaTiabie ~sa P.B.S.,·General Benefit from June 1st 1992. / The controlled assymetric nitrate plasma profile provided by Imdur affords effective protection_against angina with an easy once-daily dose without the development ofnitrate tolerance1. The product code, pack description and price for Imdur is as follows: ASTRA PRICE TO CODE PACK HOSPITALS 2826 Imdur Durules 60mg, Memory Pack of 30 $16.99 Full Product Information is on the reverse of this letter for your reference. \,' ~ 1\~ • . ' ~ @J;.~ Nyberg G. Eur J Clin Pharmacol1990;38(Suppl):Se5-S68 . 'IM~=TJRADEMARK ;t~~-- RECEIVED 6 vlf.~X I , \ Q..- • "r4-/ ,;(t1 \:;'; ~· \'P 1 -, J UN 1992 0 Postal Address TelephOi)?ug Evaluation Telex P.O. Box 131 888 7733 Branch 24461 NORTH RYDE, N.S.W., AUSTRALIA2113 Sydney Telefax 887 2879 -- / 66-78 TALAVERA ROAD, NORTH RYDE ONC·E DAILY Caution }should also be observed ff IMDUR DURULES are administered to patients w.c .