Senate Reply
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Finding the Way (WILL)
A handbook for Pakistan's Women Parliamentarians and Political Leaders LEADING THE WAY By Syed Shamoon Hashmi Women's Initiative for Learning & Wi Leadership She has and shel willl ©Search For Common Ground 2014 DEDICATED TO Women parliamentarians of Pakistan — past, present and aspiring - who remain committed in their political struggle and are an inspiration for the whole nation. And to those who support their cause and wish to see Pakistan stand strong as a This guidebook has been produced by Search For Common Ground Pakistan (www.sfcg.org/pakistan), an democratic and prosperous nation. international non-profit organization working to transform the way the world deals with conflict away from adversarial approaches and towards collaborative problem solving. The publication has been made possible through generous support provided by the U.S. Bureau of Democracy, Human Rights and Labor (DRL), under the project titled “Strengthening Women’s Political Participation and Leadership for Effective Democratic Governance in Pakistan.” The content of this publication is sole responsibility of SFCG Pakistan. All content, including text, illustrations and designs are the copyrighted property of SFCG Pakistan, and may not be copied, transmitted or reproduced, in part or whole, without the prior consent of Search For Common Ground Pakistan. Women's Initiative for Learning & Wi Leadership She has and shel willl ©Search For Common Ground 2014 DEDICATED TO Women parliamentarians of Pakistan — past, present and aspiring - who remain committed in their political struggle and are an inspiration for the whole nation. And to those who support their cause and wish to see Pakistan stand strong as a This guidebook has been produced by Search For Common Ground Pakistan (www.sfcg.org/pakistan), an democratic and prosperous nation. -

National Assembly Secretariat
NATIONAL ASSEMBLY SECRETARIAT BULLETIN OF THE ASSEMBLY (52ND SESSION) Date Tuesday, the 20th February, 2018 (Private Members’ Day) Commenced at 10.35 A.M. Adjourned at 3.05 P.M. Total working hours 4 Hours 30 Minutes Presided by 1. Sardar Ayaz Sadiq, Speaker, National Assembly of Pakistan 2. Mr. Murtaza Javed Abbasi, Deputy Speaker National Assembly of Pakistan 3. Syed Naveed Qamar, Chairperson Attendance 221 1. TILAWAT AND NAAT 1. Tilawat and Naat by Dr. Qari Muhammad Yunas LEAVE APPLICATIONS The leave applications of the Members who requested for grant of leave were read out by the honourable Mr. Speaker and leave was granted. MOTION Ms. Saira Afzal Tarar, Minister for National Health Services, Regulations and Coordination moved the following motion, which was adopted by the House:- “That under rule 288 of the Rules of Procedure and Conduct of Business in the National Assembly, 2007, the requirement of rule 51 of the said Rules in respect of Private Business, to be transacted today, the 20th February, 2018 may be suspended in order to consider Government Bills.” 1 SUPPLEMENTARY ORDERS OF THE DAY LEGISLATIVE BUSINESS 49A. MS. SAIRA AFZAL TARAR, Minister for Ms. Saira Afzal Tarar, Minister for National Health National Health Services, Regulations and Services, Regulations and Coordination moved the Coordination to move that the Bill to motion which was adopted. provide for restructuring of health services academy as a degree awarding institute [The Health Services Academy (Restructuring) Bill, 2017], as reported by the Standing Committee, be taken into consideration at once. Each clause was submitted to the Assembly and stood part of the Bill. -

February 2018 Volume 09 Issue 02 Promoting Bilateral Relations | Current Affairs | Trade & Economic Affairs | Education | Technology | Culture & Tourism ABC Certified
Monthly Magazine on National & International Political Affairs, Diplomatic Issues February 2018 Volume 09 Issue 02 Promoting Bilateral Relations | Current Affairs | Trade & Economic Affairs | Education | Technology | Culture & Tourism ABC Certified “Publishing from Pakistan, United Kingdom/EU & will be soon from UAE , Central Africa, Central Asia & Asia Pacific” Member APNS Central Media List A Largest, Widely Circulated Diplomatic Magazine | www.diplomaticfocus.org | www.diplomaticfocus-uk.com | Member Diplomatic Council /diplomaticfocusofficial /DFocusOfficial will benefit President of Indonesia H.E. Ir. H. Joko Widodo February 2018 Volume 09 Issue 02 “Publishing from Pakistan, United Kingdom/EU & will be soon from UAE ” 08 18 24 40 08 Conflicts & wars will benefit no one: President Mamnoon Hussain and President of Indonesia Ir. President of Indonesia H. Joko Widodo have agreed to work together for peace in Afghanistan and said that peace in Afghanistan is necessary for the development and progress of the region. Both countries will work together in this regard 18 Nishan-i-Imtiaz (Military) to Commander On the occasion, the President said that Pakistan and Saudi Royal Saudi Naval Forces Vice Admiral Arabia are the closest friends and the depth of their relationship Fahad Bin Abdullah Al-Ghofaily is difficult to describe in words. 24 CPEC most important initiative of our Prime Minister Shahid Khaqan said that the China Pakistan generation: PM Abbasi Economic Corridor (CPEC) is the most important initiative of our generation. “This is perhaps the most important initiative of our generation and the most visible part of the Belt and Road Initiative,” he said while addressing the inauguration ceremony of the first-ever International Gwadar Expo. -

2. MR. AKRAM MASIH GILL to Invite Attention of Minister-In-Charge Of
NATIONAL ASSEMBLY SECRETARIAT ORDERS OF THE DAY for the meeting of the National Assembly to be held on Tuesday, the 25th January, 2011 at 10.00 a.m. 1. Recitation from the Holy Quran. CALLING ATTENTION NOTICE 2. SARDAR BAHADUR AHMED KHAN SIHAR CH. ANWAR ALI CHEEMA SARDAR SHAHJEHAN YOUSAF MS. MARVI MEMON MR. AKRAM MASIH GILL to invite attention of Minister-in-Charge of the Cabinet Secretariat to a matter of urgent public importance regarding non-utilization of huge funds for rehabilitation of the flood affectees, causing grave concern amongst the public. INTRODUCTION OF BILLS 3. MR. ZAHID HAMID MR. SHAHID KHAQAN ABBASI CH. MUHAMMAD BARJEES TAHIR MR. MUHAMMAD PERVAIZ MALIK MR. MUHAMMAD HANIF ABBASI ENGR. KHURRAM DASTGIR KHAN CHAUDHRY MAHMOOD BASHIR VIRK SARDAR AYAZ SADIQ SHAIKH ROHALE ASGHAR SAHIBZADA MUHAMMAD FAZAL-E- KARIM MR. MUHAMMAD AKHTAR KHAN KANJU MS. SAIRA AFZAL TARAR MS. ANUSHA REHMAN KHAN ADVOCATE JUSTICE (RETD) IFTIKHAR AHMED CHEEMA MR. NASEER BHUTTA MR. MUHAMMAD BALIGH-UR-REHMAN CH. SAUD MAJEED BEGUM NUZHAT SADIQ DR. NELSON AZEEM MR. BILAL YASIN MR. HUMAIR HAYAT KHAN ROKHRI MR. USMAN IBRAHIM MIAN MARGHOOB AHMAD MR. MURTAZA JAVED ABBASI DR. TARIQ FAZAL CHAUDHARY MS. TAHIRA AURANGZEB to move for leave to introduce a Bill to provide for the abolition of all discretionary quotas in housing schemes in the public sector [The Abolition of Discretionary Quotas in Housing Schemes Bill, 2011]. 4. Also to introduce the Bill, if leave is granted. 5. MS. SHERRY REHMAN to move for leave to introduce a Bill to provide a law for Right to Information [The Right to Information Bill, 2011]. -

Saleem Yousuf (Pakistan 1982 to 1990)
Saleem Yousuf (Pakistan 1982 to 1990) Pakistan hosted the West Indies in 1986-87. Expecting a gruelling Test series, Imran Khan wanted known fighters in his team. Zulqarnain had kept wicket in Pakistan’s previous Test series against Sri Lanka and in the following One Day International (ODI) tournaments. Anil Dilpat was wicketkeeper in the first of two three-day tour games and in an ODI against the West Indies in October 1986. For the second three-day game, just a week before the opening Test, Imran brought in Saleem Yousuf and retained him for the series. Afterwards Imran felt his choice was vindicated: “In Salim Yousuf, Pakistan had found a courageous player and one who could handle a crisis.” When Pakistan toured the West Indies later in 1987-88, Saleem, despite a broken nose from being hit in the face in the first innings, came in at No. 9 in the second and played a vital innings of 28, adding 51 runs, to help set West Indies a testing score at Bridgetown. Wasim Akram considered Saleem “not the tidiest keeper, but he’s one of the bravest batsman I’ve ever seen.” Saleem Yousuf contributed much to Pakistan’s rise to the summit in Test cricket from 1986. Successes against West Indies, and series wins over India, England (twice), Australia and New Zealand meant that when Pakistan and the West Indies met in late 1990 the series was regarded as the unofficial world championship. Saleem played only in the first Test in that 1990 series, finishing his Test career by helping Pakistan take the lead. -
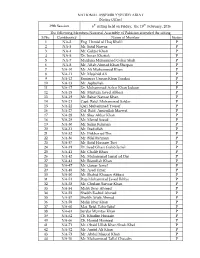
S.No. Contituency Name of Member Status 1 NA-2 Eng. Hamid Ul Haq Khalil P 2 NA-3 Mr
NATIONAL ASSEMBLY SECRETARIAT (Notice Office) 29th Session 6th sitting held on Friday, the 19th February, 2016 The following Members National Assembly of Pakistan attended the sitting S.No. Contituency Name of Member Status 1 NA-2 Eng. Hamid ul Haq Khalil P 2 NA-3 Mr. Sajid Nawaz P 3 NA-4 Mr. Gulzar Khan P 4 NA-5 Dr. Imran Khattak P 5 NA-7 Maulana Muhammad Gohar Shah P 6 NA-8 Mr. Aftab Ahmad Khan Sherpao P 7 NA-10 Mr. Ali Muhammad Khan P 8 NA-11 Mr. Mujahid Ali P 9 NA-12 Engineer Usman Khan Tarakai P 10 NA-13 Mr. Aqibullah P 11 NA-17 Dr. Muhammad Azhar Khan Jadoon P 12 NA-18 Mr. Murtaza Javed Abbasi P 13 NA-19 Mr. Babar Nawaz Khan P 14 NA-21 Capt. Retd. Muhammad Safdar P 15 NA-22 Qari Muhammad Yousaf P 16 NA-27 Col. Retd. Amirullah Marwat P 17 NA-28 Mr. Sher Akbar Khan P 18 NA-29 Mr. Murad Saeed P 19 NA-30 Mr. Salim Rehman P 20 NA-31 Mr. Ibadullah P 21 NA-32 Mr. Iftikhar ud Din P 22 NA-36 Mr. Bilal Rehman P 23 NA-37 Mr. Sajid Hussain Turi P 24 NA-39 Dr. Syed Ghazi Gulab Jamal P 25 NA-41 Mr. Ghalib Khan P 26 NA-42 Mr. Muhammad Jamal ud Din P 27 NA-43 Mr. Bismillah Khan P 28 NA-47 Mr. Qaisar Jamal P 29 NA-48 Mr. Asad Umar P 30 NA-50 Mr. Shahid Khaqan Abbasi P 31 NA-51 Raja Muhammad Javed Ikhlas P 32 NA-53 Mr. -

BWFA Holds Training Seminar
THURSDAY-MARCH 11, 2021 Sport 05 BWFA holds The life and career of Rashid Khan, Afghanistan’s cricket prodigy training seminar The life and career of Afghani- At a provincial Twenty20 young man was missing. He had id, nothing,” said Hashmi. stan cricketer Rashid Khan has seen match in Kabul, playing for a club had to dash over to Afghanistan to On one occasion, Rashid was the theatrical treatment: The cur- called Kochian, Abdulrahimzai play in a tournament. dropped due to a dispute in the tains parted one day and there he watched an unknown teenager, of This to and fro was due to team and Hashmi went to his home was. small built, batting like a dervish. Rashid’s nomadic boyhood, often to make peace. Among a cast of romantic, co- “He was opening, he scored as part of a business family along- “The next time I went to the lourful cricketers from the coun- try’s fairy tale, stood the teen prod- igy, the one player ready-made for the biggest stage. Online profiles feature Rash- id’s One-Day International (ODI) debut at the age of 17, the 25 teams he has represented in various for- mats, his admiration for Pakistan’s Shahid Afridi, that whippy, whirly action, dead-eye wrong ’un, strik- ing ability with the bat, athleticism in the field and the ICC T20 Play- er of the Decade title, even though he played in only half of it. But what about the other parts of the man that was known as Rashid Arman, the sixth of 11 brothers? The dreamer who thought he would become the fam- KABUL: The Basketball four teams from female athletes tend the international competi- ily’s first doctor? The computer Wheelchair Federation of Afghan- with each team consisting of ten tions, as well,” Zirak said. -

Shahid Afridi
Mission Statement: • To take Pakistan cricket to such a high point where it sets benchmarks at the world level. • To further enhance cricket’s mass appeal across the country by introducing high quality regional cricket at the first class level. • To promote cricketing culture by providing opportunity of participation in competitive cricket to youth at school and club level, and also to patronise and develop women’s cricket. • To total commitment to optimising talent through promotion of Coach Education Programmes and development of human resource in such vital areas as umpiring, curating and scoring etc. • To ensure excellence in governance and also to avail and improve marketing and commercial opportunities to the maximum without compromising basic ethos of the game. CONTENTS Chairman’s Report 06 Chief Operating Officer’s Report 08 TEAM GREEN: The comeback kids 10 ICC WORLD CUP 2011 Pakistan’s Report Card 14 INTERNATIONAL CRICKET REVIEW Pakistan across the three formats Only one Test loss 20 Pakistan wins 24 out of 32 ODIs 22 TWENTY20 CRICKET: Four out of five in the bag 24 Records & Milestones 2011 26 DOMESTIC CRICKET REPORT 2010-11 As many as 15 events took place during the year 32 PAKISTAN WOMEN’S CRICKET: Simply outstanding, Team Pakistan carves many a milestone 42 Game Development Plans and Activities 46 Blind/Deaf Cricket 58 Marketing Report 2011-12 62 Chief Financial Officer’s Report 2010-11 63 GHIR SA R U B Patron-in-Chief A H.E. Asif Ali Zardari, President of Pakistan esigned by B esigned by D Chairman Ch. Muhammad Zaka Ashraf nnual Report A Chief Operating Officer Subhan Ahmed Chief Financial Officer Badar M. -

November-December
PCB Highlights 1 November – 31 December 3rd Edition PCB brings Test cricket back to Pakistan The purist and most traditional format of the gentleman’s game returned to Pakistan after more than 10 years when Sri Lanka played the ICC World Test Championship fixtures in Rawalpindi and Karachi in December. For this team to put the March 2009 tragedy behind it and return twice in a space of three months was a strong and powerful statement to the world about their confidence and trust in Pakistan’s security agencies and the support to the Pakistan Cricket Board in its efforts and endeavours for the complete and uninterrupted restoration of international cricket in the country. The Sri Lanka cricket team was excellent – both on and off the field – and returned home not only after making more friends and followers than ever, but also after proving to be outstanding ambassadors of their country. They were gracious in defeat and generous in praising the hospitality and security offered to them during their Test tour. “Now I do regret not coming for the shorter formats. At that time it was a really hard decision to take because I had heard and read lots of things about Pakistan on news and social media - not positive things. But the guys who came here before gave really good comments and that’s why all the seniors decided to go and play a good Test series. Now I think I should have come and played the one-dayers,” Sri Lanka skipper Dimuth Karunaratne said. “I can say that for me it feels really safe. -
![Ruyrg ]Zgvd R `Eyvc Urj E` Wzxye](https://docslib.b-cdn.net/cover/1037/ruyrg-zgvd-r-eyvc-urj-e-wzxye-3611037.webp)
Ruyrg ]Zgvd R `Eyvc Urj E` Wzxye
/ " 5( $# %$ / %$ / / SIDISrtVUU@IB!&!!"&#S@B9IV69P99I !%! %! ' -&%1-* 2345 1+,.,1.% 01)$' +12$3 "# 9 --(39 12083(13 44 10(,!2140, (-,1403 ,3,0- 2 20,1212 ,0(1.2, &13-0- 9232-243011-4 4-12-1&-2 (-,2(0 ,?(-2(&1? ( #0 :;--, :<= >"$- $# 6 " " 236 7829: 24 7 %&' ! ' ,-,.-/0- (-,1 n a major victory for India, R Ithe International Court of Justice (ICJ) on Wednesday ruled that Pakistan must review the death sentence to Indian national Kulbhushan Jadhav, who has been sentenced to death by a Pakistani military court on charges of “espionage and terrorism”. India hailed the ICJ verdict "# with Prime Minister Narendra Modi saying “truth and justice” 0- (-,1/-0.2 have prevailed. “We welcome today’s ver- '-+ ) 1) 2 he 15 rebel Congress-JD(S) dict in the @CIJ_ICJ. Truth and - TMLAs cannot be forced to justice have prevailed. $ %&' take part in the proceedings of Congratulations to the ICJ for the ongoing Assembly session a verdict based on extensive ment directive immediately. while Speaker KR Ramesh study of facts. I am sure “This judgment validates Kumar enjoys the freedom to ! "" # $ %&' Kulbhushan Jadhav will get India’s position on the case. ' P) * "+ ,)- .%/) /' 0 ! decide on their resignations justice. Our Government will We’ll continue to work vigor- ' - - $ %&' within such time-frame as con- looks inevitable. lic mandate, setting a “terrible always work for the safety and ously for Jadhav’s early release sidered appropriate by him. While Speaker Kumar wel- judicial precedent”. welfare of every Indian,” the and return to India,” he said. access to Kulbhushan, to visit there was a three-week delay in said it was “undisputed” fact This is the crux of the comed the court decision and The court order on pleas by PM tweeted. -

December 2011
December 2011 We will have to play above our level of ability to defeat England: Taufeeq Umar We will have to exceed abilities to defeat England - Taufeeq ESPNcricinfo Dec 31, 2011 "England have been performing very well and their team combination is a very strong one with all of their players in good form," Taufeeq told PakPassion.net. "We saw last summer in England, when... Making a comeback in international cricket is harder than the debut, feels Taufeeq Umar Better.com Dec 31, 2011 While expressing his feelings to Pakpassion in an interview, Taufeeq revealed that he was under immense pressure while making his comeback against a strong team like South Africa. He said that it was easier starting an international career rather than... Taufeeq Umar is happy with Pakistan's success in the last year Better.com Dec 31, 2011 In an interview to Pakpassion, Pakistan’s opening Test batsman expressed satisfaction over his team's performance during 2011. He also expressed delight over his own performance, but said that the overall team performance is more important to... We will have to exceed abilities to defeat England - Taufiq The Daily Times Jan 01, 2011 ISLAMABAD: Taufiq Umar the Pakistan opening batsman, has said that the upcoming Test series against world No. 1 England will be a tough one for his side despite their impressive recent run. Pakistan lost only one Test in 2011, and went through the year without a series... Pakistan must play above 100% to defeat England - Taufeeq MSN Sports UK Dec 31, 2011 Taufeeq made just 18 runs in his only Test against England, in 2006, and was dropped for four years following that failure. -

Highest Single-Day Spike in JK, 162 Test Positive
9thyear of publication SrinaGAR ObsErvER Trump Threatens To ‘Close Down’ Kansal Discusses Modalities For ‘Would be great for cricket’: Pat Cummins wants Social Media After Twitter Fact Check Installation Of Smart Meters IPL in October if T20 World Cup is postponed Principal Secretary, Power Development Department, Rohit Kansal today President Donald Trump on Wednesday threatened social media companies discussed the modalities for implementation of smart metre installation A lot has been said regarding the status of the T20 World Cup in Australia and with new regulation or even shuttering a day after Twitter added fact checks project and execution of Annual Maintenance Contract (AMC) for IT the Indian Premier League. While it was decided by the BCCI that IPL is going to be to two of his tweets. The president can’t unilaterally regulate or postponed indefinitely, there has been talk on holding it in October or November close the companies, which would require action by Congress infrastructure created under R-APDRP Part-A, here at a meeting in if the World Cup is postponed. It has been reported that the T20 World Cup in or the Federal Communications Commission. But that didn’t Civil Secretariat, Jammu. Kansal, who is also Chairman of Jammu/ Australia might be postponed to 2022 owing to the COVID-19 pandemic. It is stop Trump from angrily issuing a strong warning. | Page 07 Kashmir Power Distribution Corporation Limited also ..| Page05 expected that a decision will be to be formalised when the ...| Page 08 THURSDAY, 28 MAY, 2020 05, Shawwal 1441 Hijri Published from Srinagar RNI No:JKENG/2012/43267 Vol:9 Issue No: 123 Pages:8 Rs.5.00 epaper: www.srinagarobserver.com BRIEFNEWS INDIA-ChinA BORDER stAND-OFF DAY 03: 1289 Passengers Land in JK Highest Single-Day Spike US Ready To Jammu: About 15 domestic flights with 1289 passengers on board to- day arrived at Jammu and Srinagar Mediate, Says Trump Airports on Day 3 of resumption of the air services in the Union Territo- ry of Jammu and Kashmir.