Rotavirus Vaccine Effectiveness in Hong Kong Children Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WTO GPA Statistical Report 2010-Revised 3Rd Version (
STATISTICS REPORTED UNDER ARTICLE XIX : 5 OF THE AGREEMENT ON GOVERNMENT PROCUREMENT OF THE WORLD TRADE ORGANIZATION, GENEVA PERIOD : 1.1.2010 - 31.12.2010 HONG KONG, CHINA ENTITY: HOSPITAL AUTHORITY Table of Content Part A - Reports Report No. 1 - Statistics on estimated number and value of contracts awarded for products and services, both equal or above and below the threshold value Report No. 2 - Statistics on total number and value of contracts awarded equal or above the threshold value, broken down by categories of products and services, under open, selective and limited tendering procedures Report No. 3 - Statistics on total number and value of contracts awarded under each of the cases of Article XV, paragraph 1 - limited tendering, broken down by categories of products and services Report No. 4 - Statistics on total number and value of contracts awarded for products and (where necessary) services under derogations to the Agreement Part B - Global Statistics and Details on Contracts Awarded for Products and Services to Individual Countries/Regions Part A Report No. 1 Article XIX Paragraph 5(a) Statistics on estimated number and value of contracts awarded, both equal or above and below the threshold value Tendering Procedures Equal or Above the Threshold Value Below the Threshold Value Total Construction Construction Construction Goods Services Services Goods Services Services Goods Services Services Open (No.) 99 12 - - - - 99 12 - (Value in 180,640.59 95,881.70 - - - - 180,640.59 95,881.70 - '000 SDR) Selective(No.) 1 - 2 - - - 1 - 2 (Value in 1,269.54 - 31,229.86 - - - 1,269.54 - 31,229.86 '000 SDR) Limited (No.) 76 1 - - - - 76 1 - (Value in 186,083.54 745.89 - - - - 186,083.54 745.89 - '000 SDR) Grand Total :(No.) 176 13 2 - - - 176 13 2 (Value in 367,993.67 96,627.59 31,229.86 - - - 367,993.67 96,627.59 31,229.86 '000 SDR) Notes : There may be a slight discrepancy between the sum of individual items and the total as shown in the tables owing to rounding. -

Kowloon West Cluster 九龍西聯網
Kowloon West Cluster 九龍西聯網 Section 1 : Selected Statistics Kowloon West Cluster 第一章 : 選定的統計數字 九龍西聯網 Service Capacity as at 31 March 2018 截至2018年3月31日的服務容量 No. of hospital beds 醫院病床數目 4 707 No. of psychiatric day places 精衶科日間醫院名額 303 No. of geriatric day places# 老人科日間醫院名額# 160 Service Throughputs in 2017-18 2017-18年度服務量 Inpatient and day No. of discharges and deaths 出院人次及死亡人數 297 075 inpatient services No. of live births 活產嬰兒數目 4 623 住院及日間住院服務 No. of allied health attendances 專職醫療就診人次 860 108 No. of accident and emergency attendances 急症室就診人次 483 885 No. of specialist outpatient (clinical) attendances 專科門診(臨床)就診人次 1 345 950 Ambulatory Services No. of primary care attendances 基層醫療就診人次 1 104 472 日間服務 No. of allied health attendances 專職醫療就診人次 382 862 No. of geriatric day attendances# 老人科日間醫院就診人次# 35 844 No. of psychiatric day attendances 精神科日間醫院就診人次 66 607 No. of home visits by community nurses 社康護士家訪次數 156 748 Outreach Services No. of allied health attendances 專職醫療就診人次 4 473 外展服務 No. of geriatric outreach attendances 接受老人科外展服務人次 130 668 No. of psychiatric outreach attendances 接受精神科外展服務人次 91 857 Other Services No. of radiodiagnostic attendances 放射診斷就診人次 680 025 其他服務 No. of operations done inside operating theatres 在手術室內進行的手術數目 27 156 # : Figures also include places / attendances under Integrated Discharge Support Programme for Elderly Patients (IDSP). # : 數字也包括參與離院長者綜合支援計劃的醫院名額 / 就診人次。 As at 31 Mar 2018 Hospital / Institution Specialist Outpatient Clinic General Outpatient Clinic 截至2018年3月31日 醫院 / 機構 專科門診診所 普通科門診診所 Number 數目 5 8 16 ❶ Caritas -

Report of the Steering Committee on Review of Hospital Authority
Report of the Steering Committee on Review of Hospital Authority July 2015 CONTENTS Glossary .................................................................................................................. iii Executive Summary ................................................................................................ v Chapter 1 Introduction ...................................................................................... 1 Chapter 2 Work of the Steering Committee ...................................................... 6 Chapter 3 Major Challenges Facing the Hospital Authority ............................ 9 Chapter 4 Management and Organisation Structure ....................................... 13 Chapter 5 Resource Management ................................................................... 26 Chapter 6 Staff Management .......................................................................... 42 Chapter 7 Cost Effectiveness and Service Management ................................ 59 Chapter 8 Overall Management and Control .................................................. 87 Chapter 9 Conclusion ...................................................................................... 96 Annex 1 Membership of the Steering Committee on Review of Hospital Authority ....................................................................................... 102 Annex 2 Report of the Public Engagement Programme ............................. 103 Annex 3 Clustering of Hospitals and Institutions ...................................... -

A General Brief About the Hospital Authority
Mission Statement 4. In keeping with its role, the Mission of the Hospital Authority is: · to meet the different needs of patients for public hospital services, and to improve the hospital environment for the benefit of patients; · to serve the public with care, dedication and efficiency, and to encourage community participation in the system, resulting in better care and more direct accountability to the public; · to provide rewarding, fair and challenging employment to all its staff, in an environment conducive to attracting, motivating and retaining well-qualified staff; · to advise the Government of the needs of the community for public hospital services and of the resources required to meet these needs, in order to provide adequate, efficient, effective and value for money public hospital services of the highest standards recognised internationally within the resources obtainable; and · to collaborate with other agencies and bodies in the healthcare and related fields both locally and overseas to provide the greatest benefit to the local community. Corporate Vision and Strategies 5. To realise its mission, the Hospital Authority has developed the following Corporate Vision: “The Hospital Authority will collaborate with other healthcare providers and carers in the community to create a seamless healthcare environment which will maximise healthcare benefits and meet community expectations.” 6. The Authority achieves this corporate vision by formulating a set of strategic directions every year through an extensive annual planning process, taking into account the funding position, societal expectations, Government’s healthcare policy, and the challenges in the internal and external environment. The 2 corporate vision and mission are turned into operational targets to meet the community needs for healthcare services. -
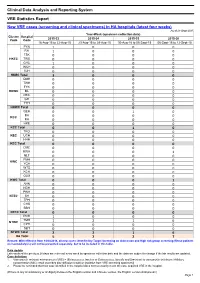
Clinical Data Analysis and Reporting System VRE Statistics Report
Clinical Data Analysis and Reporting System VRE Statistics Report New VRE cases (screening and clinical specimens) in HA hospitals (latest four weeks) As of 21-Sept-2015 Year-Week (specimen collection date) Cluster Hospital 2015-33 2015-34 2015-35 2015-36 Code Code 16-Aug-15 to 22-Aug-15 23-Aug-15 to 29-Aug-15 30-Aug-15 to 05-Sept-15 06-Sept-15 to 12-Sept-15 PYN 1 0 0 0 RH 1 0 0 0 TSK 0 0 0 0 HKEC TWE 0 0 0 0 CHC 1 0 0 0 WCH 0 0 0 0 SJH 0 0 0 0 HKEC Total 3 0 0 0 QMH 0 0 0 0 TWH 0 0 0 0 FYK 0 0 0 0 HKWC ML 0 0 0 0 DKC 0 0 0 0 GH 0 0 0 0 TYH 0 0 0 0 HKWC Total 0 0 0 0 QEH 0 0 1 0 BH KCC 0 0 0 0 KH 0 0 0 0 HKE 0 0 0 0 KCC Total 0 0 1 0 TKO 0 0 0 0 KEC UCH 0 0 0 0 HHH 0 0 0 0 KEC Total 0 0 0 0 CMC 0 0 0 0 KWH 0 0 0 1 NLT 0 0 0 0 PMH KWC 0 0 0 0 YCH 0 0 0 0 WTS 0 0 0 0 KCH 0 0 0 0 OLM 0 0 0 0 KWC Total 0 0 0 1 AHN 0 0 0 0 NDH 0 0 0 0 PWH 0 0 0 0 NTEC SH 0 0 0 0 TPH 0 0 0 0 CHS 0 0 0 0 BBH 0 0 0 0 NTEC Total 0 0 0 0 POH 1 0 0 0 TMH NTWC 1 2 1 6 CPH 0 0 0 0 SLH 0 0 0 0 NTWC Total 2 2 1 6 HA Total 5 2 2 7 Remark: With effective from 14/04/2014, all new cases identified by Target Screening on Admission and High risk group screening (Renal patients on haemodialysis) will not be presented separately, but to be included in this table. -

Event Detail (January) 01 Jan 2019 31 Dec 20
Start End CME Points Start Date End Date Event Name Organizer Venue Event Detail Time Time (Max) (January) Caritas Medical Centre American Heart Association Advance Caritas Medical Centre Resuscitation Training Centre, 5/F, Ms. Smile Pang / 01 Jan 2019 31 Dec 2019 Cardiovascular Life Support Provider 08:30 17:30 Resuscitation Training Centre Wai Oi Block, 111 Wing Hong 10.00 3408 6326 / (ACLS-P) Day 1 (Identical) (CMCRTC) Street, Shumshuipo, Kowloon, [email protected] Hong Kong Caritas Medical Centre American Heart Association Advance Caritas Medical Centre Resuscitation Training Centre, 5/F, Ms. Smile Pang / 01 Jan 2019 31 Dec 2019 Cardiovascular Life Support Provider 08:30 17:30 Resuscitation Training Centre Wai Oi Block, 111 Wing Hong 10.00 3408 6326 / (ACLS-P) Day 1 (Identical) (CMCRTC) Street, Shumshuipo, Kowloon, [email protected] Hong Kong Caritas Medical Centre American Heart Association Advance Caritas Medical Centre Resuscitation Training Centre, 5/F, Ms. Smile Pang / 01 Jan 2019 31 Dec 2019 Cardiovascular Life Support Provider 08:30 17:30 Resuscitation Training Centre Wai Oi Block, 111 Wing Hong 10.00 3408 6326 / (ACLS-P) Day 2 (Identical) (CMCRTC) Street, Shumshuipo, Kowloon, [email protected] Hong Kong Caritas Medical Centre American Heart Association Pediatric Caritas Medical Centre Resuscitation Training Centre, 5/F, Ms. Smile Pang / 01 Jan 2019 31 Dec 2019 Advanced Life Support Provider Course 08:30 17:30 Resuscitation Training Centre Wai Oi Block, 111 Wing Hong 10.00 3408 6326 / (PALS-P) Day 1 (Identical) (CMCRTC) Street, Shumshuipo, Kowloon, [email protected] Hong Kong Caritas Medical Centre American Heart Association Pediatric Caritas Medical Centre Resuscitation Training Centre, 5/F, Ms. -

Clinic Audit on Chronic Obstructive Pulmonary Disease (COPD) Management in Public Primary Care Setting: Hong Kong Experience
medRxiv preprint doi: https://doi.org/10.1101/2020.11.26.20239541; this version posted November 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . 1 Title page Clinic audit on Chronic Obstructive Pulmonary Disease (COPD) management in public primary care setting: Hong Kong experience Names of Authors: Chen Xiao Rui Catherine1, Fu Sau Nga2, Leung Wing Kit3, Ng Sze Wing Catherine4, Kwan Wing Yan Wendy5, Wong Tseng Kwong6, Chan Pang Fai6, Wong Man Ying Michelle5, Ko Wai Kit Welchie4, Liang Jun7, Hui Ming Tung Eric3, Li Yim Chu1, Luk Wan2 and Chao VK David6. Affiliations: 1. Dept. of Family Medicine and General Out Patient Clinics, Kowloon Central Cluster, Hospital Authority, Hong Kong 2. Dept. of Family Medicine and Primary Health Care, Kowloon West Cluster, Hospital Authority, Hong Kong 3. Dept. of Family Medicine, New Territories East Cluster, Hospital Authority, Hong Kong 4. Dept. of Family Medicine and Primary Health Care, Hong Kong West Cluster, Hospital Authority, Hong Kong 5. Dept. of Family Medicine and Primary Health Care, Hong Kong East Cluster, Hospital Authority, Hong Kong 6. Dept. of Family Medicine and Primary Health Care, Kowloon East Cluster, Hospital Authority, Hong Kong 7. Dept. of Family Medicine and Primary Health Care, New Territories West Cluster, Hospital Authority, Hong Kong Corresponding author: Chen XR, Catherine Email: [email protected] Correspondent address: Dept. of Family Medicine and General Outpatient Clinics, Kowloon Central Cluster, Hospital Authority, Hong Kong NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

Alert on the Rise in Candida Auris Colonisation in Hong Kong
THE GOVERNMENT OF THE HONG KONG 香 港 特 別 行 政 區 政 府 SPECIAL ADMINISTRATIVE REGION 衞 生 署 DEPARTMENT OF HEALTH 衞 生 防 護 中 心 Centre for Health Protection 感 染 控 制 處 Infection Control Branch 九 龍 亞 皆 老 街 1 4 7 C 號 地 下 G/F, 147C Argyle Street, Kowloon 本署檔號 Our Ref. : DH/ICB/P3/AT/6 來函檔號 Your Ref. : 電 話 Tel. : 圖文傳真 Fax No. : 15 October 2020 Dear Manager / Infection Control Officer of Residential Care Home, Alert on the rise in Candida auris colonisation in Hong Kong The Centre for Health Protection (CHP) of the Department of Health would like to draw your attention to the rise of Candida auris (C. auris) colonisation occurring in public hospitals in Kowloon West Cluster of the Hospital Authority (HA). Your institution is reminded to stay vigilant against the transmission of this organism among residents. In Hong Kong, C. auris colonisation is first identified in June 2019 from a patient with travel history to Switzerland. Since July 2020, outbreaks of C. auris colonisation were reported in Princess Margaret Hospital and Yan Chai Hospital. From 29 June 2020 to 9 October 2020, a total of 41 patients with C. auris colonisation have been reported to the Infection Control Branch of CHP by HA for infection control advice after discharge to residential care homes. C. auris is an emerging multi-drug resistant yeast that can cause invasive infections associated with high mortality. Treatment is relatively difficult as C. auris is resistant to many of the anti-fungal drugs. -

LC Paper No. CB(4)600/20-21(07) for Discussion on 12 March 2021
LC Paper No. CB(4)600/20-21(07) For discussion on 12 March 2021 Legislative Council Panel on Health Services Three Projects under the First Ten-year Hospital Development Plan and An Update on the First Ten-year Hospital Development Plan Purpose This paper invites Members’ comments on the following three projects under the First Ten-year Hospital Development Plan (HDP) and briefs Members on an update on the First Ten-year HDP – (a) main works for the construction of New Acute Hospital at Kai Tak Development Area at an estimated cost of $36,860.0 million in money-of-the-day (MOD) prices; (b) site formation and foundation works for the expansion of North District Hospital at an estimated cost of $2,141.0 million in MOD prices; and (c) site formation and foundation works for the expansion of Lai King Building in Princess Margaret Hospital at an estimated cost of $408.4 million in MOD prices. The total commitment sought is $39,409.4 million. Details of the above three HDP projects and the update on the First Ten-year HDP are at Enclosures 1 to 4 respectively. Background 2. In the 2016 Policy Address, the Government announced that $200 billion would be set aside for the Hospital Authority to implement the First Ten-year HDP. The First Ten-year HDP covers the redevelopment and expansion of 11 hospitals, the construction of a new acute hospital, three community health centres and one supporting services centre. Upon completion of all the projects under the First Ten-year HDP, it will provide more than 6 000 additional bed spaces, 94 additional operating theatres and increased capacity of specialist outpatient clinics and general outpatient clinics. -

Special Visiting Arrangement Resumes in Infirmary Hospitals
Special Visiting Arrangement Resumes in Infirmary Hospitals The following is issued on behalf of the Hospital Authority: The Hospital Authority (HA) today (October 31) announced that special visiting arrangement in infirmary hospitals will be implemented beginning next Wednesday (November 4). The special visiting arrangement will begin next week and initially cover eight hospitals (as appended table). Ward staff will begin contacting patients' family members today for scheduling the visits. Family members are not required to call the wards for booking themselves. Each patient will be allocated a one-hour session by one registered visitor each week, with the arrangement of the hospitals. The HA spokesperson said, "the HA has implemented special visiting arrangement in June but suspended upon the third wave of the epidemic." "With the ongoing COVID-19 pandemic around the world, the Emergency Response Level will likely be maintained for a while. Amid easing situation locally, both the HA Central Command Committee and HA Central Committee on Infectious Disease and Emergency Responses have deliberated and assessed the infection control risk associated with visiting arrangement. It was agreed that special visiting arrangement could be first resumed in infirmary hospitals." "Long-stay patients rely more heavily on the support from family members both psychologically and in their daily lives. The hospitals concerned have not received any COVID-19 patients and crowd control measures for visitors are physically viable." The arrangement will be adjusted -

Day Surgery in Hong Kong
The Journal of One Day Surgery | 97 Featured Day Surgery Units: Day Surgery in Hong Kong JOE KM FAN, WAI LUN LAW & WAI KEI YUEN Keywords: Day surgery facilities; International practice Background Concerning surgical training in Hong Kong, there is no designated module for day surgery. The application of day Medical infrastructure in Hong Kong is mainly surgery requires careful selection of cases and special care government funded, although charity groups subsidise by specialists. In addition, the physical setup and scale of some hospitals in addition to central funding from the day surgery facilities varies greatly among the different Government. The whole territory, which has a population hospital clusters (Figures 1 & 2): some are well equipped of eight million, is served by seven hospital clusters. with operating theatres and nurse specialist for Despite the fact that some hospitals have started a scheme perioperative care; whereas some only comprise a small of “Self-Financed Items” in which patients are required to side-ward with a few beds with nurses being deployed from pay the cost of expensive drugs, such as new other surgical wards during normal working hours. chemotherapeutic agents and special instruments for some operative procedures, an average patient pays as little as US$12 per day for in-hospital charge, which includes the room as well as all investigations and treatments during the hospital stay. The remaining cost is fully covered by the Hospital Authority of the Hong Kong Special Administrative Region (HKSAR), China. Under these circumstances, there is no apparent motivation for patients to be treated as day cases, as the cost for finding a care-giver or for travelling may be much more than staying as an inpatient for several more days. -

LC Paper No. CB(2)2162/14-15(01)
LC Paper No. CB(2)2162/14-15(01) Annex Response to Information Requested at Health Service Panel Meeting on 15 July 2013 Introduction The public healthcare system is the cornerstone of Hong Kong’s healthcare system. The Government will uphold its commitment to public healthcare system and will continue to deploy resources to expand our public healthcare infrastructure through building new hospitals and improving existing hospital facilities to cope with the ever-increasing demand for medical services. 2. The Hospital Authority (HA) is a major healthcare service provider in Hong Kong, currently managing 42 public hospitals/institutions with a total of some 27 000 hospital beds, 47 specialist outpatient clinics (SOPC) and 73 general outpatient clinics (GOPC). These facilities are organised into seven clusters according to geographical locations. 3. There is a high demand for HA services. Its throughputs in 2013-14 amounted to about 1.57 million inpatient and day inpatient1 discharge episodes, 2.24 million Accident & Emergency attendances, 7.04 million specialist outpatient (clinical) attendances, 2.33 million allied health (outpatient) attendances, 6.10 million primary care attendances, and 1.99 million community outreach visits. Development of Hospital Clustering 4. When HA took over the management of the public hospital system in 1991, the hospitals were disorganized and poorly coordinated. For example, some of the major acute hospitals were then supported by up to five to six “district” hospitals widely dispersed in the territory providing convalescent care. Moreover, 1 In HA, day inpatients refer to those who are admitted into hospitals for non-emergency treatment and who are discharged within the same day.