(Spermatophyta, Bryophyta, and Lichenes) of the Estonian Dry Grassland Flora
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gymnosperms the MESOZOIC: ERA of GYMNOSPERM DOMINANCE
Chapter 24 Gymnosperms THE MESOZOIC: ERA OF GYMNOSPERM DOMINANCE THE VASCULAR SYSTEM OF GYMNOSPERMS CYCADS GINKGO CONIFERS Pinaceae Include the Pines, Firs, and Spruces Cupressaceae Include the Junipers, Cypresses, and Redwoods Taxaceae Include the Yews, but Plum Yews Belong to Cephalotaxaceae Podocarpaceae and Araucariaceae Are Largely Southern Hemisphere Conifers THE LIFE CYCLE OF PINUS, A REPRESENTATIVE GYMNOSPERM Pollen and Ovules Are Produced in Different Kinds of Structures Pollination Replaces the Need for Free Water Fertilization Leads to Seed Formation GNETOPHYTES GYMNOSPERMS: SEEDS, POLLEN, AND WOOD THE ECOLOGICAL AND ECONOMIC IMPORTANCE OF GYMNOSPERMS The Origin of Seeds, Pollen, and Wood Seeds and Pollen Are Key Reproductive SUMMARY Innovations for Life on Land Seed Plants Have Distinctive Vegetative PLANTS, PEOPLE, AND THE Features ENVIRONMENT: The California Coast Relationships among Gymnosperms Redwood Forest 1 KEY CONCEPTS 1. The evolution of seeds, pollen, and wood freed plants from the need for water during reproduction, allowed for more effective dispersal of sperm, increased parental investment in the next generation and allowed for greater size and strength. 2. Seed plants originated in the Devonian period from a group called the progymnosperms, which possessed wood and heterospory, but reproduced by releasing spores. Currently, five lineages of seed plants survive--the flowering plants plus four groups of gymnosperms: cycads, Ginkgo, conifers, and gnetophytes. Conifers are the best known and most economically important group, including pines, firs, spruces, hemlocks, redwoods, cedars, cypress, yews, and several Southern Hemisphere genera. 3. The pine life cycle is heterosporous. Pollen strobili are small and seasonal. Each sporophyll has two microsporangia, in which microspores are formed and divide into immature male gametophytes while still retained in the microsporangia. -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Experimental and Cytological Studies on Plant Species
Biologiske Skrifter udgivet af Det Kongelige Danske Videnskabernes Selskab Bind 11, nr. 6 Biol. Skr. Dan. Vid. Selsk. 11, no. 6 (1963) EXPERIMENTAL AND CYTOLOGICAL STUDIES ON PLANT SPECIES VIII. RACIAL DIFFERENTIATION IN AMPHI-ATLANTIC VISCARIA ALPINA BY TYGE W. BOCHER København 1963 i kommission hos Ejnar Munksgaard Det Kongelige Danske Videnskabernes Selskab udgiver følgende pub likationsrækker : The Royal Danish Academy of Sciences and Letters issues the fol lowing series of publications: Bibliographical Abbreviation Oversigt over Selskabets Virksomhed (8°) Overs. Dan. Vid. Selsk. (Annual in Danish) Historisk-fllosofiske Meddelelser (8°) Hist. Filos. Medd. Dan. Vid. Selsk. Historisk-filosofiske Skrifter (4°) Hist. Filos. Skr. Dan. Vid. Selsk. (History, Philology, Philosophy, Archeology, Art History) Matematisk-fysiske Meddelelser (8°) Mat. Fys. Medd. Dan. Vid. Selsk. Matematisk-fysiske Skrifter (4°) Mat. Fys. Skr. Dan. Vid. Selsk. (Mathematics, Physics, Chemistry, Astronomy, Geology) Biologiske Meddelelser (8°) Biol. Medd. Dan. Vid. Selsk. Biologiske Skrifter (4°) Biol. Skr. Dan. Vid. Selsk. (Botany, Zoology, General Biology) Selskabets sekretariat og postadresse: Dantes Plads 5, København V. The address of the secretariate of the Academy is: Det Kongelige Danske Videnskabernes Selskab, Dantes Plads 5, Kbbenhavn V, Denmark. Selskabets kommissionær: E jn a r Mu n k sg a a r d ’s Forlag, Nørregade 6, København K. The publications are sold by the agent of the Academy: E jn a r Mu n k sg a a r d , Publishers, 6 Norregade, Kbbenhavn K, Denmark. BIOLOGISKE SKRIFTER UDGIVET AF DET KGL. DANSKE VIDENSKABERNES SELSKAB BIND 11 KØBENHAVN I KOMMISSION HOS EJNAR MUNKSGAARD 1959-63 INDHOLD Side 1. Foged, Niels: Diatoms from Afghanistan. -
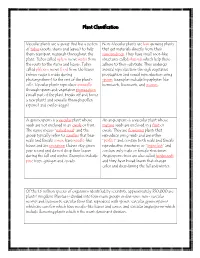
Plant Classification
Plant Classification Vascular plants are a group that has a system Non-Vascular plants are low growing plants of tubes (roots, stems and leaves) to help that get materials directly from their them transport materials throughout the surroundings. They have small root-like plant. Tubes called xylem move water from structures called rhizoids which help them the roots to the stems and leaves. Tubes adhere to their substrate. They undergo called phloem move food from the leaves asexual reproduction through vegetative (where sugar is made during propagation and sexual reproduction using photosynthesis) to the rest of the plant’s spores. Examples include bryophytes like cells. Vascular plants reproduce asexually hornworts, liverworts, and mosses. through spores and vegetative propagation (small part of the plant breaks off and forms a new plant) and sexually through pollen (sperm) and ovules (eggs). A gymnosperm is a vascular plant whose An angiosperm is a vascular plant whose seeds are not enclosed in an ovule or fruit. mature seeds are enclosed in a fruit or The name means “naked seed” and the ovule. They are flowering plants that group typically refers to conifers that bear reproduce using seeds and are either male and female cones, have needle-like “perfect” and contain both male and female leaves and are evergreen (leaves stay green reproductive structures or “imperfect” and year round and do not drop their leaves contain only male or female structures. during the fall and winter. Examples include Angiosperm trees are also called hardwoods pine trees, ginkgos and cycads. and they have broad leaves that change color and drop during the fall and winter. -

Doctorat De L'université De Toulouse
En vue de l’obt ention du DOCTORAT DE L’UNIVERSITÉ DE TOULOUSE Délivré par : Université Toulouse 3 Paul Sabatier (UT3 Paul Sabatier) Discipline ou spécialité : Ecologie, Biodiversité et Evolution Présentée et soutenue par : Joeri STRIJK le : 12 / 02 / 2010 Titre : Species diversification and differentiation in the Madagascar and Indian Ocean Islands Biodiversity Hotspot JURY Jérôme CHAVE, Directeur de Recherches CNRS Toulouse Emmanuel DOUZERY, Professeur à l'Université de Montpellier II Porter LOWRY II, Curator Missouri Botanical Garden Frédéric MEDAIL, Professeur à l'Université Paul Cezanne Aix-Marseille Christophe THEBAUD, Professeur à l'Université Paul Sabatier Ecole doctorale : Sciences Ecologiques, Vétérinaires, Agronomiques et Bioingénieries (SEVAB) Unité de recherche : UMR 5174 CNRS-UPS Evolution & Diversité Biologique Directeur(s) de Thèse : Christophe THEBAUD Rapporteurs : Emmanuel DOUZERY, Professeur à l'Université de Montpellier II Porter LOWRY II, Curator Missouri Botanical Garden Contents. CONTENTS CHAPTER 1. General Introduction 2 PART I: ASTERACEAE CHAPTER 2. Multiple evolutionary radiations and phenotypic convergence in polyphyletic Indian Ocean Daisy Trees (Psiadia, Asteraceae) (in preparation for BMC Evolutionary Biology) 14 CHAPTER 3. Taxonomic rearrangements within Indian Ocean Daisy Trees (Psiadia, Asteraceae) and the resurrection of Frappieria (in preparation for Taxon) 34 PART II: MYRSINACEAE CHAPTER 4. Phylogenetics of the Mascarene endemic genus Badula relative to its Madagascan ally Oncostemum (Myrsinaceae) (accepted in Botanical Journal of the Linnean Society) 43 CHAPTER 5. Timing and tempo of evolutionary diversification in Myrsinaceae: Badula and Oncostemum in the Indian Ocean Island Biodiversity Hotspot (in preparation for BMC Evolutionary Biology) 54 PART III: MONIMIACEAE CHAPTER 6. Biogeography of the Monimiaceae (Laurales): a role for East Gondwana and long distance dispersal, but not West Gondwana (accepted in Journal of Biogeography) 72 CHAPTER 7 General Discussion 86 REFERENCES 91 i Contents. -

Molecular Phylogeny of Subtribe Artemisiinae (Asteraceae), Including Artemisia and Its Allied and Segregate Genera Linda E
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications in the Biological Sciences Papers in the Biological Sciences 9-26-2002 Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera Linda E. Watson Miami University, [email protected] Paul E. Bates University of Nebraska-Lincoln, [email protected] Timonthy M. Evans Hope College, [email protected] Matthew M. Unwin Miami University, [email protected] James R. Estes University of Nebraska State Museum, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/bioscifacpub Watson, Linda E.; Bates, Paul E.; Evans, Timonthy M.; Unwin, Matthew M.; and Estes, James R., "Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera" (2002). Faculty Publications in the Biological Sciences. 378. http://digitalcommons.unl.edu/bioscifacpub/378 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications in the Biological Sciences by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. BMC Evolutionary Biology BioMed Central Research2 BMC2002, Evolutionary article Biology x Open Access Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera Linda E Watson*1, Paul L Bates2, Timothy M Evans3, -

Alien Flora of Europe: Species Diversity, Temporal Trends, Geographical Patterns and Research Needs
Preslia 80: 101–149, 2008 101 Alien flora of Europe: species diversity, temporal trends, geographical patterns and research needs Zavlečená flóra Evropy: druhová diverzita, časové trendy, zákonitosti geografického rozšíření a oblasti budoucího výzkumu Philip W. L a m b d o n1,2#, Petr P y š e k3,4*, Corina B a s n o u5, Martin H e j d a3,4, Margari- taArianoutsou6, Franz E s s l7, Vojtěch J a r o š í k4,3, Jan P e r g l3, Marten W i n t e r8, Paulina A n a s t a s i u9, Pavlos A n d r i opoulos6, Ioannis B a z o s6, Giuseppe Brundu10, Laura C e l e s t i - G r a p o w11, Philippe C h a s s o t12, Pinelopi D e l i p e t - rou13, Melanie J o s e f s s o n14, Salit K a r k15, Stefan K l o t z8, Yannis K o k k o r i s6, Ingolf K ü h n8, Hélia M a r c h a n t e16, Irena P e r g l o v á3, Joan P i n o5, Montserrat Vilà17, Andreas Z i k o s6, David R o y1 & Philip E. H u l m e18 1Centre for Ecology and Hydrology, Hill of Brathens, Banchory, Aberdeenshire AB31 4BW, Scotland, e-mail; [email protected], [email protected]; 2Kew Herbarium, Royal Botanic Gardens Kew, Richmond, Surrey, TW9 3AB, United Kingdom; 3Institute of Bot- any, Academy of Sciences of the Czech Republic, CZ-252 43 Průhonice, Czech Republic, e-mail: [email protected], [email protected], [email protected], [email protected]; 4Department of Ecology, Faculty of Science, Charles University, Viničná 7, CZ-128 01 Praha 2, Czech Republic; e-mail: [email protected]; 5Center for Ecological Research and Forestry Applications, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain, e-mail: [email protected], [email protected]; 6University of Athens, Faculty of Biology, Department of Ecology & Systematics, 15784 Athens, Greece, e-mail: [email protected], [email protected], [email protected], [email protected], [email protected]; 7Federal Environment Agency, Department of Nature Conservation, Spittelauer Lände 5, 1090 Vienna, Austria, e-mail: [email protected]; 8Helmholtz Centre for Environmental Research – UFZ, Department of Community Ecology, Theodor-Lieser- Str. -

European Glacial Relict Snails and Plants: Environmental Context of Their Modern Refugial Occurrence in Southern Siberia
bs_bs_banner European glacial relict snails and plants: environmental context of their modern refugial occurrence in southern Siberia MICHAL HORSAK, MILAN CHYTRY, PETRA HAJKOV A, MICHAL HAJEK, JIRI DANIHELKA, VERONIKA HORSAKOV A, NIKOLAI ERMAKOV, DMITRY A. GERMAN, MARTIN KOCI, PAVEL LUSTYK, JEFFREY C. NEKOLA, ZDENKA PREISLEROVA AND MILAN VALACHOVIC Horsak, M., Chytry, M., Hajkov a, P., Hajek, M., Danihelka, J., Horsakov a,V.,Ermakov,N.,German,D.A.,Ko cı, M., Lustyk, P., Nekola, J. C., Preislerova, Z. & Valachovic, M. 2015 (October): European glacial relict snails and plants: environmental context of their modern refugial occurrence in southern Siberia. Boreas, Vol. 44, pp. 638–657. 10.1111/bor.12133. ISSN 0300-9483. Knowledge of present-day communities and ecosystems resembling those reconstructed from the fossil record can help improve our understanding of historical distribution patterns and species composition of past communities. Here, we use a unique data set of 570 plots explored for vascular plant and 315 for land-snail assemblages located along a 650-km-long transect running across a steep climatic gradient in the Russian Altai Mountains and their foothills in southern Siberia. We analysed climatic and habitat requirements of modern populations for eight land-snail and 16 vascular plant species that are considered characteristic of the full-glacial environment of central Europe based on (i) fossil evidence from loess deposits (snails) or (ii) refugial patterns of their modern distribu- tions (plants). The analysis yielded consistent predictions of the full-glacial central European climate derived from both snail and plant populations. We found that the distribution of these 24 species was limited to the areas with mean annual temperature varying from À6.7 to 3.4 °C (median À2.5 °C) and with total annual precipitation vary- ing from 137 to 593 mm (median 283 mm). -

New Taxa of Sorbus from Bohemia (Czech Republic)
Verh.© Zool.-Bot. Zool.-Bot. Ges. Österreich, Ges. Austria;Österreich download 133 unter www.biologiezentrum.at(1996): 319-345 New taxa of Sorbus from Bohemia (Czech Republic) M iloslav Ko v a n da Three species of Sorbus are described as new: S. rhodanthera (one Station in W Bohemia) and S. gemella (one Station in W Central Bohemia) belonging to the S. latifolia agg. and S. quernea (two stations in Central Bohemia) of the S. hybrida agg. Basic data on their karyology, morphology, Variation, relation- ships, geographical distribution, ecology and ecobiology are provided. Also described is one primary hybrid, S. X abscondita (S. aucuparia L. X S. danu- bialis [JÄv.] P rodan) o f the S. hybrida agg. Kovanda M., 1996: Neue Sorbus-Taxa aus Böhmen (Tschechische Republik). Drei Sorbus- Arten werden als neu beschrieben: S. rhodanthera (ein Fundort in W-Böhmen) und S. gemella (ein Fundort in W-Mittelböhmen) der S. latifolia agg. und S. quernea (zwei Fundorte in Mittelböhmen) der S. hybrida agg. Grundlegende Daten zur Karyologie, Morphologie, Variation, Verwandtschaft, geographischen Verbreitung, Ökologie, Phytozönologie und Ökobiologie werden präsentiert. Weiters wird eine Primärhybride, S. X abscondita (S. aucuparia L. X S. danubialis [JÄv.] Prodan) der 5. hybrida agg. beschrieben. Keywords: Sorbus, Bohemia, chromosome numbers, morphological Variation, geographical distribution, ecology, phytocenology, ecobiology, interspecific hybridization. Introduction Interspecific hybridization with concomitant polyploidy and apomixis is an acknowledged vehicle of speciation in Sorbus (e.g. Liu efo r s 1953, 1955, Ko v a n d a 1961, Challice & Ko v a n d a 1978, Ja nk un & Ko v a n da 1986, 1987, 1988, Kutzelnigg 1994). -

5. Tribe CICHORIEAE 菊苣族 Ju Ju Zu Shi Zhu (石铸 Shih Chu), Ge Xuejun (葛学军); Norbert Kilian, Jan Kirschner, Jan Štěpánek, Alexander P
Published online on 25 October 2011. Shi, Z., Ge, X. J., Kilian, N., Kirschner, J., Štěpánek, J., Sukhorukov, A. P., Mavrodiev, E. V. & Gottschlich, G. 2011. Cichorieae. Pp. 195–353 in: Wu, Z. Y., Raven, P. H. & Hong, D. Y., eds., Flora of China Volume 20–21 (Asteraceae). Science Press (Beijing) & Missouri Botanical Garden Press (St. Louis). 5. Tribe CICHORIEAE 菊苣族 ju ju zu Shi Zhu (石铸 Shih Chu), Ge Xuejun (葛学军); Norbert Kilian, Jan Kirschner, Jan Štěpánek, Alexander P. Sukhorukov, Evgeny V. Mavrodiev, Günter Gottschlich Annual to perennial, acaulescent, scapose, or caulescent herbs, more rarely subshrubs, exceptionally scandent vines, latex present. Leaves alternate, frequently rosulate. Capitulum solitary or capitula loosely to more densely aggregated, sometimes forming a secondary capitulum, ligulate, homogamous, with 3–5 to ca. 300 but mostly with a few dozen bisexual florets. Receptacle naked, or more rarely with scales or bristles. Involucre cylindric to campanulate, ± differentiated into a few imbricate outer series of phyllaries and a longer inner series, rarely uniseriate. Florets with 5-toothed ligule, pale yellow to deep orange-yellow, or of some shade of blue, including whitish or purple, rarely white; anthers basally calcarate and caudate, apical appendage elongate, smooth, filaments smooth; style slender, with long, slender branches, sweeping hairs on shaft and branches; pollen echinolophate or echinate. Achene cylindric, or fusiform to slenderly obconoidal, usually ribbed, sometimes compressed or flattened, apically truncate, attenuate, cuspi- date, or beaked, often sculptured, mostly glabrous, sometimes papillose or hairy, rarely villous, sometimes heteromorphic; pappus of scabrid [to barbellate] or plumose bristles, rarely of scales or absent. -

Bryophyte Diversity and Vascular Plants
DISSERTATIONES BIOLOGICAE UNIVERSITATIS TARTUENSIS 75 BRYOPHYTE DIVERSITY AND VASCULAR PLANTS NELE INGERPUU TARTU 2002 DISSERTATIONES BIOLOGICAE UNIVERSITATIS TARTUENSIS 75 DISSERTATIONES BIOLOGICAE UNIVERSITATIS TARTUENSIS 75 BRYOPHYTE DIVERSITY AND VASCULAR PLANTS NELE INGERPUU TARTU UNIVERSITY PRESS Chair of Plant Ecology, Department of Botany and Ecology, University of Tartu, Estonia The dissertation is accepted for the commencement of the degree of Doctor philosophiae in plant ecology at the University of Tartu on June 3, 2002 by the Council of the Faculty of Biology and Geography of the University of Tartu Opponent: Ph.D. H. J. During, Department of Plant Ecology, the University of Utrecht, Utrecht, The Netherlands Commencement: Room No 218, Lai 40, Tartu on August 26, 2002 © Nele Ingerpuu, 2002 Tartu Ülikooli Kirjastuse trükikoda Tiigi 78, Tartu 50410 Tellimus nr. 495 CONTENTS LIST OF PAPERS 6 INTRODUCTION 7 MATERIAL AND METHODS 9 Study areas and field data 9 Analyses 10 RESULTS 13 Correlation between bryophyte and vascular plant species richness and cover in different plant communities (I, II, V) 13 Environmental factors influencing the moss and field layer (II, III) 15 Effect of vascular plant cover on the growth of bryophytes in a pot experiment (IV) 17 The distribution of grassland bryophytes and vascular plants into different rarity forms (V) 19 Results connected with nature conservation (I, II, V) 20 DISCUSSION 21 CONCLUSIONS 24 SUMMARY IN ESTONIAN. Sammaltaimede mitmekesisus ja seosed soontaimedega. Kokkuvõte 25 < TÄNUSÕNAD. Acknowledgements 28 REFERENCES 29 PAPERS 33 2 5 LIST OF PAPERS The present thesis is based on the following papers which are referred to in the text by the Roman numerals. -

The “Plant Drosophila”: E. B. Babcock, the Genus Crepis, and the Evolution of a Genetics Research Program at Berkeley, 1915–1947
HSNS3903_02 6/26/09 11:04 AM Page 300 ∗ VASSILIKI BETTY SMOCOVITIS The “Plant Drosophila”: E. B. Babcock, the Genus Crepis, and the Evolution of a Genetics Research Program at Berkeley, 1915–1947 The student of genetics must be ready to resort to the use of any living organism that gives promise of revealing the natural laws upon which the future science of breeding will be grounded. E. B. Babcock, 19131 The Crepis investigations carried on by the Babcock group are the American evolutionary investigations that seem to have attracted the largest attention outside of America next to the Drosophila investigations. One reason for this is their wider systematical aspect. Jens Clausen, 19342 *Departments of Zoology and History, Affiliate in Botany, University of Florida, Bartram Hall, Gainesville, FL 32611; [email protected]fl.edu, [email protected]fl.edu. The following abbreviations are used: APS, American Philosophical Society Library, Philadel- phia, PA; CAS, California Academy of Sciences, San Francisco, CA; CIW, Carnegie Institution of Washington; CP, Carnegie Papers, Missouri Botanical Garden Archives, St. Louis, MO; EBB, Ernest Brown Babcock Papers, TBL; JHB, Journal of the History of Biology; JAJ, James Angus Jenkins Papers, TBL; RG, Rockefeller Foundation Archives, Sleepy Hollow, NY, http://www.rockarch.org/collections/rf/; TBL, The Bancroft Library, University of California, Berkeley; UC, University of California; UCGD, University of California, Berkeley, Genetics Department, APS. Interviews with G. Ledyard Stebbins are transcribed and in the author’s possession. 1. “Division of Genetics: Report to the Director of the Experiment Station, 11 June 1913,” EBB, Folder The Division of Genetics of the Department of Agriculture.