Density-Dependent Migration in an Amphiura Filiformis (Amphiuridae, Echinodermata) Infaunal Population
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Monitoring Program for Biodiversity and Non-Indigenous Species in Egypt
UNITED NATIONS ENVIRONMENT PROGRAM MEDITERRANEAN ACTION PLAN REGIONAL ACTIVITY CENTRE FOR SPECIALLY PROTECTED AREAS National monitoring program for biodiversity and non-indigenous species in Egypt PROF. MOUSTAFA M. FOUDA April 2017 1 Study required and financed by: Regional Activity Centre for Specially Protected Areas Boulevard du Leader Yasser Arafat BP 337 1080 Tunis Cedex – Tunisie Responsible of the study: Mehdi Aissi, EcApMEDII Programme officer In charge of the study: Prof. Moustafa M. Fouda Mr. Mohamed Said Abdelwarith Mr. Mahmoud Fawzy Kamel Ministry of Environment, Egyptian Environmental Affairs Agency (EEAA) With the participation of: Name, qualification and original institution of all the participants in the study (field mission or participation of national institutions) 2 TABLE OF CONTENTS page Acknowledgements 4 Preamble 5 Chapter 1: Introduction 9 Chapter 2: Institutional and regulatory aspects 40 Chapter 3: Scientific Aspects 49 Chapter 4: Development of monitoring program 59 Chapter 5: Existing Monitoring Program in Egypt 91 1. Monitoring program for habitat mapping 103 2. Marine MAMMALS monitoring program 109 3. Marine Turtles Monitoring Program 115 4. Monitoring Program for Seabirds 118 5. Non-Indigenous Species Monitoring Program 123 Chapter 6: Implementation / Operational Plan 131 Selected References 133 Annexes 143 3 AKNOWLEGEMENTS We would like to thank RAC/ SPA and EU for providing financial and technical assistances to prepare this monitoring programme. The preparation of this programme was the result of several contacts and interviews with many stakeholders from Government, research institutions, NGOs and fishermen. The author would like to express thanks to all for their support. In addition; we would like to acknowledge all participants who attended the workshop and represented the following institutions: 1. -

High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project
High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project AEA Technology, Environment Contract: W/35/00632/00/00 For: The Department of Trade and Industry New & Renewable Energy Programme Report issued 30 August 2002 (Version with minor corrections 16 September 2002) Keith Hiscock, Harvey Tyler-Walters and Hugh Jones Reference: Hiscock, K., Tyler-Walters, H. & Jones, H. 2002. High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project. Report from the Marine Biological Association to The Department of Trade and Industry New & Renewable Energy Programme. (AEA Technology, Environment Contract: W/35/00632/00/00.) Correspondence: Dr. K. Hiscock, The Laboratory, Citadel Hill, Plymouth, PL1 2PB. [email protected] High level environmental screening study for offshore wind farm developments – marine habitats and species ii High level environmental screening study for offshore wind farm developments – marine habitats and species Title: High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project. Contract Report: W/35/00632/00/00. Client: Department of Trade and Industry (New & Renewable Energy Programme) Contract management: AEA Technology, Environment. Date of contract issue: 22/07/2002 Level of report issue: Final Confidentiality: Distribution at discretion of DTI before Consultation report published then no restriction. Distribution: Two copies and electronic file to DTI (Mr S. Payne, Offshore Renewables Planning). One copy to MBA library. Prepared by: Dr. K. Hiscock, Dr. H. Tyler-Walters & Hugh Jones Authorization: Project Director: Dr. Keith Hiscock Date: Signature: MBA Director: Prof. S. Hawkins Date: Signature: This report can be referred to as follows: Hiscock, K., Tyler-Walters, H. -

Preliminary Mass-Balance Food Web Model of the Eastern Chukchi Sea
NOAA Technical Memorandum NMFS-AFSC-262 Preliminary Mass-balance Food Web Model of the Eastern Chukchi Sea by G. A. Whitehouse U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Alaska Fisheries Science Center December 2013 NOAA Technical Memorandum NMFS The National Marine Fisheries Service's Alaska Fisheries Science Center uses the NOAA Technical Memorandum series to issue informal scientific and technical publications when complete formal review and editorial processing are not appropriate or feasible. Documents within this series reflect sound professional work and may be referenced in the formal scientific and technical literature. The NMFS-AFSC Technical Memorandum series of the Alaska Fisheries Science Center continues the NMFS-F/NWC series established in 1970 by the Northwest Fisheries Center. The NMFS-NWFSC series is currently used by the Northwest Fisheries Science Center. This document should be cited as follows: Whitehouse, G. A. 2013. A preliminary mass-balance food web model of the eastern Chukchi Sea. U.S. Dep. Commer., NOAA Tech. Memo. NMFS-AFSC-262, 162 p. Reference in this document to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA. NOAA Technical Memorandum NMFS-AFSC-262 Preliminary Mass-balance Food Web Model of the Eastern Chukchi Sea by G. A. Whitehouse1,2 1Alaska Fisheries Science Center 7600 Sand Point Way N.E. Seattle WA 98115 2Joint Institute for the Study of the Atmosphere and Ocean University of Washington Box 354925 Seattle WA 98195 www.afsc.noaa.gov U.S. DEPARTMENT OF COMMERCE Penny. S. Pritzker, Secretary National Oceanic and Atmospheric Administration Kathryn D. -

Full Text in Pdf Format
MARINE ECOLOGY PROGRESS SERIES Published September 17 Mar Ecol Prog Ser - I P P Accumulation of polychlorinated biphenyls by the inf aunal brittle stars Amphiura filiformis and A. chiajei: effects of eutrophication and selective feeding Jonas S. Gunnarsson*,Mattias Sköld** Department of Marine Ecology. Göteborg University, Kristineberg Marine Research Station. 450 34 Fiskebäckskil, Sweden ABSTRACT: Polychlonnated biphenyl (PCB) accumulation by the 2 brittle stars Amphiura filiformis and A. chjajei was studied in a laboratory experiment and in the field. In the laboratory study. the fate of '4C-2,2',4,4'-tetrachlorobiphenyl(TCB) was determined in benthic microcosn~s,with and without the addition of phytoplankton (Phaeodactylum tricornutum) Added phytoplankton was rapidly miner- alised and stimulated an increased dissolved organic carbon content in the water-column and bacterial production on the sediment surface. TCB uptake by the brittle stars was significantly higher in the microcosms enriched with phytoplankton. Differences in TCB concentrations were still significant after normalisation to lipid content, suggesting that selective feeding rather than equilibnum partitioning was the cause of the increased TCB burden. Treatment effects were more apparent in body (disk) tissue, than in the arm fraction of the bnttle stars, in agreement with the lipid content of the tissues. No difference in total organic carbon, total nitrogen or TCB concentrations of the sediment surface was detected. In the field, ophuroids and sediment cores were collected at a coastal urban estuary off the city of Göteborg, Sweden, and at an offshore station in the Kattegat Sea. Surn-PCBs of sediment and brittle stars were ca 3 times higher at the coastal station than at the offshore station. -

Key to the Common Shallow-Water Brittle Stars (Echinodermata: Ophiuroidea) of the Gulf of Mexico and Caribbean Sea
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/228496999 Key to the common shallow-water brittle stars (Echinodermata: Ophiuroidea) of the Gulf of Mexico and Caribbean Sea Article · January 2007 CITATIONS READS 10 702 1 author: Christopher Pomory University of West Florida 34 PUBLICATIONS 303 CITATIONS SEE PROFILE All content following this page was uploaded by Christopher Pomory on 21 May 2014. The user has requested enhancement of the downloaded file. All in-text references underlined in blue are added to the original document and are linked to publications on ResearchGate, letting you access and read them immediately. 1 Key to the common shallow-water brittle stars (Echinodermata: Ophiuroidea) of the Gulf of Mexico and Caribbean Sea CHRISTOPHER M. POMORY 2007 Department of Biology, University of West Florida, 11000 University Parkway, Pensacola, FL 32514, USA. [email protected] ABSTRACT A key is given for 85 species of ophiuroids from the Gulf of Mexico and Caribbean Sea covering a depth range from the intertidal down to 30 m. Figures highlighting important anatomical features associated with couplets in the key are provided. 2 INTRODUCTION The Caribbean region is one of the major coral reef zoogeographic provinces and a region of intensive human use of marine resources for tourism and fisheries (Aide and Grau, 2004). With the world-wide decline of coral reefs, and deterioration of shallow-water marine habitats in general, ecological and biodiversity studies have become more important than ever before (Bellwood et al., 2004). Ecological and biodiversity studies require identification of collected specimens, often by biologists not specializing in taxonomy, and therefore identification guides easily accessible to a diversity of biologists are necessary. -

Bioluminescence in the Ophiuroid Amphiura Filiformis (O.F. Müller
Echinoderm Research 2001, Féral & David (eds.) ©2003 Swets <S Zeitlinger, Lisse, ISBN 90 5809 528 2 Bioluminescence in the ophiuroidAmphiura filiformis (O.F. Müller, 1776) is not temperature dependant 3 7 2 4 4 O. Bruggeman, S. Dupont, J. Mallefet Laboratoire de Physiologie Animale & Centre de Recherche sur la Biodiversité, Université catholique de Louvain, Louvain-la-Neuve, Belgium R. Bannister & M.C, Thorndyke Bourne Laboratory, Royal Halloway College, University o f London, UK ABSTRACT: Influence of temperature on the bioluminescence of the ophiuroidAmphiura filiformis was experimentally tested. Amputated arms from individuals acclimated at 6° or 14°C were incubated at five dif ferent temperatures ranging from 6° to 14°C for 2 hours before KC1 stimulation. Light response comparisons do not reveal any difference neither for the two temperatures of acclimation nor for the five temperatures of incubation. Therefore, the temperature does not affect the studied parameters of the light emission. KEYWORDS: Amphiura filiformis, ophiuroid, bio luminescence, temperature. 1 INTRODUCTION natural environment of this species, the aim of this work was to characterize the short-term (few hours) Amphiura filiformis (O.F. Müller, 1776) is a brittle star and long-term (a month) effect of temperature on the with a total diameter of 15 cm. It lives in the mud of bio luminescence A.of filiformis. North European coasts and in the Mediterranean Sea. It constitutes an important fish food resources since it has been estimated that it provides up to 301 metric 2 MATERIAL AND METHODS tons of biomass per year (Nilsson & Sköld 1996). The species is also bioluminescent and arms emit blueSpecimens o fAmphiura filiformis were collected in the light when individuals are mechanically stimulatedGullmar Fjord (Fiskebackskill, Sweden) by Petersen (Emson & Herring 1985; Mallefet pers. -

National Monitoring Program for Biodiversity and Non-Indigenous Species in Egypt
National monitoring program for biodiversity and non-indigenous species in Egypt January 2016 1 TABLE OF CONTENTS page Acknowledgements 3 Preamble 4 Chapter 1: Introduction 8 Overview of Egypt Biodiversity 37 Chapter 2: Institutional and regulatory aspects 39 National Legislations 39 Regional and International conventions and agreements 46 Chapter 3: Scientific Aspects 48 Summary of Egyptian Marine Biodiversity Knowledge 48 The Current Situation in Egypt 56 Present state of Biodiversity knowledge 57 Chapter 4: Development of monitoring program 58 Introduction 58 Conclusions 103 Suggested Monitoring Program Suggested monitoring program for habitat mapping 104 Suggested marine MAMMALS monitoring program 109 Suggested Marine Turtles Monitoring Program 115 Suggested Monitoring Program for Seabirds 117 Suggested Non-Indigenous Species Monitoring Program 121 Chapter 5: Implementation / Operational Plan 128 Selected References 130 Annexes 141 2 AKNOWLEGEMENTS 3 Preamble The Ecosystem Approach (EcAp) is a strategy for the integrated management of land, water and living resources that promotes conservation and sustainable use in an equitable way, as stated by the Convention of Biological Diversity. This process aims to achieve the Good Environmental Status (GES) through the elaborated 11 Ecological Objectives and their respective common indicators. Since 2008, Contracting Parties to the Barcelona Convention have adopted the EcAp and agreed on a roadmap for its implementation. First phases of the EcAp process led to the accomplishment of 5 steps of the scheduled 7-steps process such as: 1) Definition of an Ecological Vision for the Mediterranean; 2) Setting common Mediterranean strategic goals; 3) Identification of an important ecosystem properties and assessment of ecological status and pressures; 4) Development of a set of ecological objectives corresponding to the Vision and strategic goals; and 5) Derivation of operational objectives with indicators and target levels. -

The Echinoderm Fauna of Turkey with New Records from the Levantine Coast of Turkey
Proc. of middle East & North Africa Conf. For Future of Animal Wealth THE ECHINODERM FAUNA OF TURKEY WITH NEW RECORDS FROM THE LEVANTINE COAST OF TURKEY Elif Özgür1, Bayram Öztürk2 and F. Saadet Karakulak2 1Faculty of Fisheries, Akdeniz University, TR-07058 Antalya, Turkey 2İstanbul University, Faculty of Fisheries, Ordu Cad.No.200, 34470 Laleli- Istanbul, Turkey Corresponding author e-mail: [email protected] ABSTRACT The echinoderm fauna of Turkey consists of 80 species (two Crinoidea, 22 Asteroidea, 18 Ophiuroidea, 20 Echinoidea and 18 Holothuroidea). In this study, seven echinoderm species are reported for the first time from the Levantine coast of Turkey. These are, five ophiroid species; Amphipholis squamata, Amphiura chiajei, Amphiura filiformis, Ophiopsila aranea, and Ophiothrix quinquemaculata and two echinoid species; Echinocyamus pusillus and Stylocidaris affinis. Turkey is surrounded by four seas with different hydrographical characteristics and Turkish Straits System (Çanakkale Strait, Marmara Sea and İstanbul Strait) serve both as a biological corridor and barrier between the Aegean and Black Seas. The number of echinoderm species in the coasts of Turkey also varies due to the different biotic environments of these seas. There are 14 echinoderm species reported from the Black Sea, 19 species from the İstanbul Strait, 51 from the Marmara Sea, 71 from the Aegean Sea and 42 from the Levantine coasts of Turkey. Among these species, Asterias rubens, Ophiactis savignyi, Diadema setosum, and Synaptula reciprocans are alien species for the Turkish coasts. Key words: Echinodermata, new records, Levantine Sea, Turkey. Cairo International Covention Center , Egypt , 16 - 18 – October , (2008), pp. 571 - 581 Elif Özgür et al. -
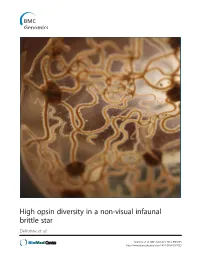
Amphiura Filiformis, We First Highlighted a Blue-Green Light Sensitivity Using a Behavioural Approach
High opsin diversity in a non-visual infaunal brittle star Delroisse et al. Delroisse et al. BMC Genomics 2014, 15:1035 http://www.biomedcentral.com/1471-2164/15/1035 Delroisse et al. BMC Genomics 2014, 15:1035 http://www.biomedcentral.com/1471-2164/15/1035 RESEARCH ARTICLE Open Access High opsin diversity in a non-visual infaunal brittle star Jérôme Delroisse1*, Esther Ullrich-Lüter2, Olga Ortega-Martinez3, Sam Dupont3, Maria-Ina Arnone4, Jérôme Mallefet5 and Patrick Flammang1 Abstract Background: In metazoans, opsins are photosensitive proteins involved in both vision and non-visual photoreception. Echinoderms have no well-defined eyes but several opsin genes were found in the purple sea urchin (Strongylocentrotus purpuratus) genome. Molecular data are lacking for other echinoderm classes although many species are known to be light sensitive. Results: In this study focused on the European brittle star Amphiura filiformis, we first highlighted a blue-green light sensitivity using a behavioural approach. We then identified 13 new putative opsin genes against eight bona fide opsin genes in the genome of S. purpuratus. Six opsins were included in the rhabdomeric opsin group (r-opsins). In addition, one putative ciliary opsin (c-opsin), showing high similarity with the c-opsin of S. purpuratus (Sp-opsin 1), one Go opsin similar to Sp-opsins 3.1 and 3.2, two basal-branch opsins similar to Sp-opsins 2 and 5, and two neuropsins similar to Sp-opsin 8, were identified. Finally, two sequences from one putative RGR opsin similar to Sp-opsin 7 were also detected. Adult arm transcriptome analysis pinpointed opsin mRNAs corresponding to one r-opsin, one neuropsin and the homologue of Sp-opsin 2. -

Echinodermata, Ophiuroidea)
Vol. 16: 105–113, 2012 AQUATIC BIOLOGY Published online July 19 doi: 10.3354/ab00435 Aquat Biol Slow arm regeneration in the Antarctic brittle star Ophiura crassa (Echinodermata, Ophiuroidea) Melody S. Clark*, Terri Souster British Antarctic Survey, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 0ET, UK ABSTRACT: Regeneration of arms in brittle stars is thought to proceed slowly in low temperature environments. Here a survey of natural arm damage and arm regeneration rates is documented in the Antarctic brittle star Ophiura crassa. This relatively small ophiuroid, a detritivore found amongst red macroalgae, displays high levels of natural arm damage and repair. This is largely thought to be due to ice damage in the shallow waters it inhabits. The time scale of arm regener- ation was measured in an aquarium-based 10 mo experiment. There was a delayed regeneration phase of 7 mo before arm growth was detectable in this species. This is 2 mo longer than the longest time previously described, which was in another Antarctic ophiuroid, Ophionotus victo- riae. The subsequent regeneration of arms in O. crassa occurred at a rate of approximately 0.16 mm mo−1. To date, this is the slowest regeneration rate known of any ophiuroid. The confir- mation that such a long delay before arm regeneration occurs in a second Antarctic species pro- vides strong evidence that this phenomenon is yet another characteristic feature of Southern Ocean species, along with deferred maturity, slowed growth and development rates. It is unclear whether delayed initial regeneration phases are adaptations to, or limitations of, low temperature environments. -

Fisheries Management Controls for Dab in the North Sea
Fisheries management controls for dab in The North Sea Auteurs: D.C.M. Miller and R. Verkempynck IMARES report C040/16 Fisheries management controls for dab in the North Sea Author(s): D.C.M. Miller and R. Verkempynck Client: Ministerie van Economische Zaken Directie ELVV T.a.v. Ir. H.R. Offringa Postbus 20401 2500 EK Den Haag Project nummer: BO 4311810002 BAS-code BO-20-010-076 Publication date: 12 April 2016 IMARES Wageningen UR IJmuiden, April 2016 IMARES report C040/16 D.C.M. Miller and R. Verkempynck, 2016. Fisheries management controls for dab in the North Sea; Wageningen, IMARES Wageningen UR (University & Research centre), IMARES report C040/16. © 2016 IMARES Wageningen UR IMARES, institute of Stichting DLO The Management of IMARES is not responsible for resulting damage, as well as is registered in the Dutch trade for damage resulting from the application of results or research obtained by record nr. 09098104, IMARES, its clients or any claims related to the application of information found BTW nr. NL 806511618 within its research. This report has been made on the request of the client and is wholly the client's property. This report may not be reproduced and/or published partially or in its entirety without the express written consent of the client. A_4_3_2 V21 IMARES report C040/16 | 2 van 31 Table of Contents Table of Contents ..................................................................................................................... 3 Summary ............................................................................................................................... -

Ophiuroidea: Amphilepidida: Amphiuridae), from Geoje Island, Korea
-페이지 연속.(가능한한 짝수되게). -표 선: 맨위,맨아래 1pt / 중간 0.3pt -표 내부여백 : 선 있는 곳 2, 선 없는 곳 0.7 -et al. Journal of Species Research 9(3):273-279, 2020 A newly recorded brittle star, Amphiura (Amphiura) digitula (H.L. Clark, 1911) (Ophiuroidea: Amphilepidida: Amphiuridae), from Geoje Island, Korea Taekjun Lee1 and Sook Shin1,2 1Marine Biological Resource Institute, Sahmyook University, Seoul 01795, Republic of Korea 2Department of Animal Biotechnology and Resource, Sahmyook University, Seoul 01795, Republic of Korea *Correspondent: [email protected] We describe a newly recorded brittle star to South Korea, Amphiura (Amphiura) digitula (H.L. Clark, 1911), that was collected from Geoje Island, at a depth of 47 m. The species is characterized by a small disk, covered by numerous fine scales, small radial shields that are wider than long, a small stumpy hook at the distal end of the radial shield, two tooth papilla, two adoral shield spines, 2nd adoral shield spine longer than other, tapered dramatically toward dull tip, five arms with four proximal arm spines, and two tentacle scales. We also obtained a 657 bp sequence from COI gene and the amplified sequence matched the general DNA barcoding region. The NJ and ML phylogenetic analyses revealed A. (A.) digitula as monophyletic in the Amphiura clade. This species is clearly distinguished from other Amphiura species by morphological characteristics and the mitochondrial COI sequence, and thus represents the sixth Amphiura species reported to occur in Korea. Keywords: Echinodermata, mitochondrial COI, morphology, ophiuroid, taxonomy Ⓒ 2020 National Institute of Biological Resources DOI:10.12651/JSR.2020.9.3.273 INTRODUCTION al., 2016; Boissin et al., 2017; Knott et al., 2018; Paw- son, 2018).