Application of a Mass Balance Partitioning Model of Ra-226, Pb-210 and Po-210 to Freshwater Lakes and Streams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Newsletterlake Kashagawigamog Organization
WINTER 2019 NEWSLETTERLake Kashagawigamog Organization THE BEAUTY OF WINTER ON KASHAGAWIGAMOG! Lake Kashagawigamog Organization LAKE KASHAGAWIGAMOG ORGANIZATION NEWSLETTER LAKE KASHAGAWIGAMOG ORGANIZATION NEWSLETTER Greetings From The President LKO Annual SEPTIC Winter 2019 INSPECTIONS As autumn fades and winter arrives it is time to reflect on the FOCA, and local politicians to handle General summer of 2019 on Lake Kashagawigamog. The ice finally the many issues relating to the lake. receded at the beginning of May and after a wet spring we We need your help! First, we need you to Meeting The preservation of our natural environment is headed into a drought which meant sunny weekends through speak to your friends and neighbours on most of the summer. Good planning and water management an endeavour that we all share. To this end it the lake to get them to become members of the LKO. Together we will be the small individual efforts that will resulted in us having above average water levels for the season. have a more powerful voice to get things done. SATURDAY JUNE 27, 2020 make the difference, natural shorelines and The LKO had a busy summer. We started with a very successful Second, we need volunteers. They say that many hands make responsible management of our onsite Love your Lake Seminar, our Annual General Meeting, a Cottage 9:00 A.M. light work. Well, we have light work but we need more hands to wastewater. The little steps can pay big Succession Planning seminar with Soyers Lake and FOCA (www. get it done. We want to continue to put on educational and fun Location: To Be Determined foca.on.ca), and another successful Kash Bash. -

Miskwabi Area Watershed Plan * Are Unique in That They Form the Head Waters of the Burnt River System
ATVing MISKWABI Litter Waterskiing AREA Acid Rain Motor Swimming WATERSHED Canoeing Boating PLAN Renting Roads Logging An Integral Vision for the Fishing Invasive Species Lakes of Long, Miskwabi, Negaunee and Wenona Hunting Water Nutrients • Everything interconnects. Levels Hiking • Everything affects every other thing. Wildlife Forests Streams Septic • Everything brings change to Systems all. Shorelines • Everything influences the Global entire web of the watershed. Warming Personal Watercraft Nothing is separate! Snowmobiling TABLE OF CONTENTS INTRODUCTION................................................... 2 PHYSICAL ELEMENTS ............................................. 16 SOILS ............................................................................ 16 VALUES AND MISSION ................................................. 4 MINERALS & AGGREGATES .......................................... 16 THE WATERSHED PLAN SURVEY ............................... 4 STEEP SLOPES............................................................... 16 MISSION STATEMENT ............................................... 5 NARROW WATER BODIES ............................................ 16 SUMMARY OF STEWARDSHIP ACTION PLAN ....... 6 SOCIAL ELEMENTS ................................................. 48 LAKE DESCRIPTION ............................................ 10 SOCIAL CULTURE .......................................................... 48 HISTORY .................................................................. 10 TRANQUILITY & NIGHT SKIES ...................................... -

Trent Assessment Report
TRENT CONSERVATION COALITION SOURCE PROTECTION REGION Approved Trent Assessment Report Approved October 1, 2014 Volume 1 of 3 Effective Janurary 1, 2015 Updated February 15, 2018 Trent Source Protection Areas: Crowe Valley Source Protection Area Kawartha-Haliburton Source Protection Area Lower Trent Source Protection Area Otonabee-Peterborough Source Protection Area Made possible through the support of the Government of Ontario www.trentsourceprotection.on.ca This Assessment Report was prepared on behalf of the Trent Conservation Coalition Source Protection Committee under the Clean Water Act, 2006. TRENT CONSERVATION COALITION SOURCE PROTECTION COMMITTEE Jim Hunt (Chair) Municipal The Trent Conservation Coalition Source Dave Burton Protection Committee is a locally based Rob Franklin (Bruce Craig to June 2011) committee, comprised of 28 Dave Golem representatives from municipal Rosemary Kelleher‐MacLennan government, First Nations, the Gerald McGregor commercial/industrial/agriculture sectors, Mary Smith and other interests. The Committee’s Richard Straka ultimate role is to develop a Source Protection Plan that establishes policies for Commercial/Industrial preventing, reducing, or eliminating threats Monica Berdin, Recreation/Tourism to sources of drinking water. In developing Edgar Cornish, Agriculture the plan, the committee members are Kerry Doughty, Aggregate/Mining Robert Lake, Economic Development committed to the following: Glenn Milne, Agriculture . Basing policies on the best available Bev Spencer, Agriculture science, and -

Eat Play Stay
Canoe Longspur Source Lake Canisbay Lake Lake Lake Jack Lake Bonita Lake Smoke Tanamakoon Lake of Tea Lake Lake Lake Cache Two Rivers Little Island Lake Provoking Swan Lake Lake Lake Norway 1 Lake Fork Lake Ragged Lake 3 3 2 4 Oxtongue Lake Oxtongue Lake Algonquin To North Bay Highlands (125 km) Clinto ALGONQUIN Lake Selected Features Tock Lake 1 Point of Interest 5 2 Kawagama 1 Trail Head 6 Lake 127 Lake of Bays Dorset 1 1 Point of Interest & Trail Head PROVINCIAL Raven HALIBURTON FOREST Lake FROST CENTRE AND WILDLIFE RESERVE AREA Little Kennisis Lake Red Pine 5 Nunikani 7 Lake Lake Kennisis Lake Macdonald Lake Follow to Sherborne Lake St. Nora Little PARK Highway 62 South 8 Lake Big Hawk 9 Redstone continue to Lake Lake Marsden Lake 8 Peterson Rd Little Hawk turns into Halls Lake Kabakwa Lake 4 County Road 10 Lake Redstone (Elephant Lake Rd) Halls Percy Kushog Lake Lake (55 km) POKER LAKES Lake Fort Irwin AREA Lake 58 mins 12 Haliburton 10 West Guilford Lake Moose Lake 11 Maple Kingscote Lake Big East Pine Eagle Lake Lake Eagle Lake Lake Beech 26 Lake Boshkung Lake Lake 10 22 9 13 Little Boshkung Carnarvon Crotchet Lake 6 Lake 14 Benoir Lake Twelve Mile Dysart et al 6 2 Lake 16 Minden Fishtail Lake 1 23 Soyers 7 Follow to Hills Lake g Haliburton Horseshoe mo Spruce Lake Highway 62 North ga Drag Lake Lake wi Elephant Lake 25 ga Kennibik Lake Mountain a 15 continue to Big Trout ash Lake ke K 27 17 Lake 18 La Highway 127 North 18 Grace 15 21 Miskwabi Farquhar follow to Lake 20 22 Lake Lake Harcourt Bob Lake Highway 60 West Canning Donald -

The Beautiful Lake: a Bi‐National Biodiversity Conservation Strategy for Lake Ontario
The Beautiful Lake A Bi‐national Biodiversity Conservation Strategy for Lake Ontario Prepared by the Lake Ontario Bi‐national Biodiversity Conservation Strategy Working Group In cooperation with the U.S. – Canada Lake Ontario Lakewide Management Plan April 2009 ii The name "Ontario" comes from a native word, possibly "Onitariio" or "Kanadario", loosely translated as "beautiful" or "sparkling" water or lake. (Government of Ontario 2008) The Beautiful Lake: A Bi‐national Biodiversity Conservation Strategy for Lake Ontario April 2009 Prepared by: Lake Ontario Biodiversity Strategy Working Group In co‐operation with: U.S. – Canada Lake Ontario Lake‐wide Management Plan Acknowledgements Funding for this initiative was provided by the Great Lakes National Program Office and Region 2 of the U.S. Environmental Protection Agency, the Canada‐Ontario Agreement Respecting the Great Lakes Basin Ecosystem, The Nature Conservancy and The Nature Conservancy of Canada. This conservation strategy presented in this report reflects the input of over 150 experts representing 53 agencies including Conservation Authorities, universities, and NGOs (see Appendix A.1). In particular, the authors acknowledge the guidance and support of the project Steering Committee: Mark Bain (Cornell University), Gregory Capobianco (New York Department of State), Susan Doka (Department of Fisheries and Oceans), Bonnie Fox (Conservation Ontario), Michael Greer (U.S. Army Corps of Engineers), Frederick Luckey (U.S. Environmental Protection Agency), Jim MacKenzie (Ontario Ministry of Natural Resources), Mike McMurtry (Ontario Ministry of Natural Resources), Joseph Makarewicz (SUNY‐Brockport), Angus McLeod (Parks Canada), Carolyn O’Neill (Environment Canada1), Karen Rodriquez (U.S. Environmental Protection Agency) and Tracey Tomajer (New York State Department of Environmental Conservation). -

Sturgeon Lake Watershed Characterization Report
Sturgeon Lake Watershed Characterization Report 2014 ii STURGEON LAKE WATERSHED CHARACTERIZATION REPORT KAWARTHA CONSERVATION About Kawartha Conservation A plentiful supply of clean water is a key component of our natural infrastructure. Our surface and groundwater resources supply our drinking water, maintain property values, sustain an agricultural industry and support tourism. Kawartha Conservation is the local environmental agency through which we can protect our water and other natural resources. Our mandate is to ensure the conservation, restoration and responsible management of water, land and natural habitats through programs and services that balance human, environmental and economic needs. We are a non-profit environmental organization, established in 1979 under the Ontario Conservation Authorities Act (1946). We are governed by the six municipalities that overlap the natural boundaries of our watershed and voted to form the Kawartha Region Conservation Authority. These municipalities include the City of Kawartha Lakes, Township of Scugog (Region of Durham), Township of Brock (Region of Durham), the Municipality of Clarington (Region of Durham), Township of Cavan Monaghan, and the Municipality of Trent Lakes. iv STURGEON LAKE WATERSHED CHARACTERIZATION REPORT KAWARTHA CONSERVATION Acknowledgements This Watershed Characterization Report was prepared by the Technical Services Department team of Kawartha Conservation with considerable support from other staff. The following individuals have written sections of the report: Alexander -

Real Estate Guide Waterfront
Issue 8 • August 1, 2013 STATE GUIDE STATE E REAL Real Estate Guide WATERFRONT Marj & John Parish Sales Representatives Living, Loving, CONDOS and Selling Life in the Highlands! RE/MAXNORTH COUNTRY REALTY INC, BROKERAGE 1-800-465-2984 Offi ce: 705-457-1011 Ext: 226 RESIDENTIAL [email protected] www.johnparish.net Home: 705-457-2986 Check out our listings on pg 2 Cover photo courtesy of Elizabeth Thompson LAND Haliburton O ce Minden O ce Kinmount O ce 705-457-2414 705-286-1234 705-488-3077 197 Highland Street 12621 Highway 35 3613 Cty Road 121 www.royallepagelakeso aliburton.ca COTTAGES RE/MAX NORTH COUNTRY Marj & John 1-800-465-2984 REALTY INC, BROKERAGE OFFICE: 705-457-1011 EXT: 226 1-800-465-2984 Offi ce: 705-457-1011 EXT: 226 [email protected] [email protected] Parish WWW.JOHNPARISH.NET www.johnparish.net Home: 705-457-2986 Sales Representatives WELCOME TO SUNSET POINT KENNISIS LAKE - $769,900 Welcome to Sunset Point on prestigious Kennisis Lake! This is it, the perfect blend of peace & serenity. Its all yours on this private, level, Algonquin style paradise on 2 Separate Deeded Lots! The winterized pine interior cottage sits on one lot & a dry boathouse that sleeps 5 on the other. Your cottage is approached via a circular driveway off a year round twp road. Wander through your forest of windswept pines & hemlock to the rocky point waterfront. Go for a swim off the dock in deep crystal clear water or wade in the sandy cove, then sit by the fi re as you watch the most amazing sunsets from your front row seat and enjoy this million dollar big lake view! For a more private sunset experience sit on the upper deck hidden in the trees that was designed specifi cally for sunset viewing. -

AGENDA Regular Council Meeting
The Municipality of Dysart et al AGENDA Regular Council Meeting August 27, 2012 9:00 a.m. Page 1. ADOPTION OF AGENDA 2. DISCLOSURE OF PECUNIARY INTEREST 3. ADOPTION OF MINUTES FROM PREVIOUS MEETING 5-22 4. FINANCE DEPARTMENT 23 July 2012 Cheque Summary. 24-25 Monthly Revenue/Expense Report as at July 31, 2012. 26 Metered Sewage Report for July 2012. 27-28 s.357/358 Tax Adjustments. 29 Thunder Bay & Area Disaster Relief Fund Re: Request for Financial Support. 5. DELEGATIONS 9:30 a.m. - Gail Stelter, Colourfest Coordinator Re: Colourfest 2012. 10:00 a.m. - Greg Bishop, Items P-14, 15, 18 and 19. 6. FIRE DEPARTMENT 30 Fire Chief Report. 7. ROADS DEPARTMENT 31 Roads Update. 32-35 Roads Needs Study. 36 Ontario Good Roads Association - Alert Re: Minister of Transportation Agrees to "Open Up" MMS Regulations. 8. ENVIRONMENT 37 Recycled Material Report - July 2012. Page 1 of 251 Page 8. ENVIRONMENT 38 Landfill Tipping Fees Report - July. 9. PARKS AND RECREATION DEPARTMENT 39 Parks and Recreation Update. 10. BUILDING AND BY-LAW DEPARTMENT 40 Building Permit Report to July 31, 2012. 41-42 By-law Department Report - July 2012. 11. ADMINISTRATION 43-45 Environment and Green Energy Committee Re: Minutes of July 3, 2012 Meeting. 46-49 Ground Mount Solar Installations. 50-52 Housing and Business Development Committee Re: Minutes of July 3, 2012 Meeting. 53-56 Haliburton Highlands Museum Board Re: a) Minutes of July 3, 2012 Meeting. b) Request for Installation of Railing for Front Ramp. 57-60 Glebe Park Committee Re: Minutes of June 12, 2012 Meeting. -

Lake Plan February 2015
Gull Lake - Lake Plan February 2015 Acknowledgements Prepared by: The Gull Lake - Lake Plan Steering Committee, consisting of: • Mike Thorne - Committee Chair • Don Drouillard - GLCA Lake Steward • Bruce McClennan-Water Resource Specialist • Richard Newman - GLCA Vice President • Larry Clarke - Councillor, Township of Minden Hills • With assistance from French Planning Services Inc. (Randy French and Julia Sutton) Sponsored by: • Gull Lake Cottagers Association and the generous donations and volunteer time of many individuals. Special thanks to: The following people that helped to gather and present background information for various Sections of this Report: • Heather Reid Past Executive Director, U-Links • Emily Grubb (U-links, Trent University, research project) • Don Drouillard (interpretation of data) • Helga Sonnenberg (in-depth study of lake water and source water, benthics, water quality, and toxicology) • Glen Bonham and David Flowers (fish community) • Dave Bonham (Light Pollution) • Bill Chambers (invasive species and climate change) • Marilyn Hagerman (Lake History) • Don Bell (Boating) • Kathy Hamilton (Physical Elements • Bill Lett (Land Development) • Carol McClennan (Land Use) Also a Special Thanks to Maxxam Laboratories for their analysis of the Benthic and Chemical samples and to Stantec Environmental Consultants for the interpretation of the benthic samples. Also a special thanks to Glen Bonham for his detailed review of the draft document for spelling and grammar. As well, 2 key reference documents that provided background support in the development of the Lake Plan should be noted, namely; the Lake Stewards Handbook (Haliburton Highlands Stewardship Council); and the Lake Planning Handbook for Community Groups (FOCA, French Planning Services, and Haliburton Highlands Stewardship Council). i Table of Contents Page Executive Summary ................................................................................................................... -
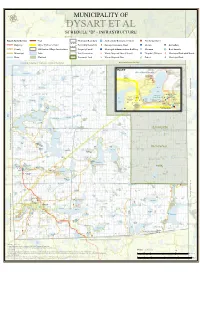
Infrastructure
d[ d[ d[ d[ d[ d[ d[ d[ d[ d[ d[ d[ d[ d[ d[ d[ d[ d[ d[ µ MUNICIPALITY OF DYSART ET AL SCHEDULE "D" - INFRASTRUCTURE d[ Road Jurisdiction Trail Municipal Boundary !Z Auditorium/Recreation Centre «¬ Fire Department 12 (! Highway Major Hydro Corridor Township Boundary ³ Sewage Treatment Plant IH Library . Art Gallery (! County Haliburton Village Service Area Property Parcel ÆX Municipal Administration Building ²¸ Museum d[ Boat Launch Municipal Lake Lot/Concession L Waste Disposal Sites (Closed) ÆP Hospital/Heliport Ý Municipal Park with Beach d[ River Wetland Provincial Park L Waste Disposal Sites 5 SchooÝl Ý Municipal Park Township of Algonquin Highlands, County of Haliburton AlgLonquin Provincial Park INSET Glebe Park Ý Haliburton Highlands Museum ²¸ Kawagama Lake Fleming College - Head Lake Wildcat Lake Haliburton School Trail South of the Arts g Wildcat Slipper Lake n i Lake 5 s i p Head Lake Trail p Stocking i Lake N Havelock Lake Head f o t c Lake i r t ³ s HAVELOCK Haliburton County i Library - D Stuart W. Haliburton Head Lake/ Dysart Branch Grass Baker Highlands Rotary Beach Park Ý Johnson Secondary Sam Lake Elementary Haliburton County IH Drag River Goodwin Lake Ý . School 5 Slick Rail Trail Trail Buckhorn Lake EYRE School d[ Lake Park Rails End 5 J. Douglas A.J. Larue CLYDE Community 5 Hodgson Gallery and Kelly Arts Centre ÆX Centre Lake Elementary !Z Little !Z n School Kennisis Haliburton Curling Club «¬ o t Lake PÆ and Squash Club r u Bone Lake b i l a Red H Ý Pine f Little Eyre Lake Lake o Clean Clean Lake y Kennisis Lake -

Parks Canada Agency Drag Lake Dams Rehabilitation
PARKS CANADA AGENCY DRAG LAKE DAMS REHABILITATION SPECIFICATIONS Rev. 2 - Issued for Tender June 17, 2016 Prepared by KGS Group TABLE OF CONTENTS Section Number Title 01 11 00 Summary of Work 01 14 00 Work Restrictions 01 22 01 Measurement and Payment 01 31 19 Project Meetings 01 33 00 Submittal Procedures 01 35 29 Health and Safety Requirements 01 35 43 Environmental Procedures 01 45 00 Quality Control 01 52 00 Construction Facilities 01 56 00 Temporary Barriers and Enclosures 01 71 00 Examination and Preparation 01 74 11 Cleaning 01 77 00 Closeout Procedures 01 78 00 Closeout Submittals 02 41 19 Selective Structure Demolition 03 10 00 Concrete Forming and Accessories 03 20 00 Concrete Reinforcing 03 30 00 Cast-in-Place Concrete 03 37 26 Underwater Placed Concrete 05 12 23 Structural Steel 05 51 29 Metal Stairs 31 11 00 Clearing and Grubbing 31 68 13 Post-Tensioned Anchor 35 20 21 Cofferdam and Dewatering 14-1538-002 Section 01 11 00 June 2016 SUMMARY OF WORK Drag Lake Dam Rehabilitation Page 1 Part 1 General 1.1 RELATED REQUIREMENTS .1 Section 01 14 00: Work Restriction .2 Section 01 33 00: Submittal Procedures .3 Section 01 35 43: Environmental Procedures .4 Section 01 45 00: Quality Control .5 Section 01 71 00: Examination and Preparation 1.2 DEFINITIONS .1 Departmental Representative: Parks Canada Agency (PCA) .2 Consultant: KGS Group .3 Contractor: General Contractor who assumes the role of Constructor 1.3 WORK COVERED BY CONTRACT DOCUMENTS .1 Work of this Contract comprises rehabilitation of Drag Lake North Dam and South Dam, located on the Drag River in the Burnt River watershed at the outlet of Outlet Bay (on Drag Lake), near Haliburton, Ontario. -

Water Management Plan
Water Management Plan For Waterpower Drag Lake Generating Station March 2005 1 WATER MANAGEMENT PLAN FOR WATERPOWER for the Drag Lake Generating Station on the Drag River OMNR Bancroft District, Southern Region Algonquin Power Fund “Canada” Incorporated for the 10-year period April 1, 2005 to March 31, 2015 In submitting this plan, I declare that this water management plan for waterpower has been prepared in accordance with the Ontario Ministry of Natural Resources, Water Management Planning Guidelines for Waterpower, as approved by the Minister of Natural Resources on May 14, 2002. _______________________________________________________________________ David Kerr, Principal, Algonquin Power Fund “Canada” Incorporated Date I have authority to bind the corporation. I certify that this water management plan has been prepared in accordance with the Ontario Ministry of Natural Resources, Water Management Planning Guidelines for Waterpower, as approved by the Minister of Natural Resources on May 14, 2002, and that direction from other sources, relevant policies and other obligations have been considered. I recommend this plan be approved for implementation. ________________________________________________________________________ Monique Rolf von den Baumen-Clark, District Manager, Bancroft District Date Ministry of Natural Resources Approved by: __________________________________ Ron Running, Regional Director, Southern Region Ministry of Natural Resources In 1994, MNR finalized its Statement of Environmental Values (SEV) under the Environmental Bill of Rights. The SEV is a document that describes how the purposes of the EBR are to be considered whenever decisions that might significantly affect the environment are made in the ministry. During the development of this water management plan, the ministry has considered its SEV. 2 This water management plan (WMP) sets out legally enforceable provisions for the management of flows and levels on this river within the values and conditions identified in the WMP.