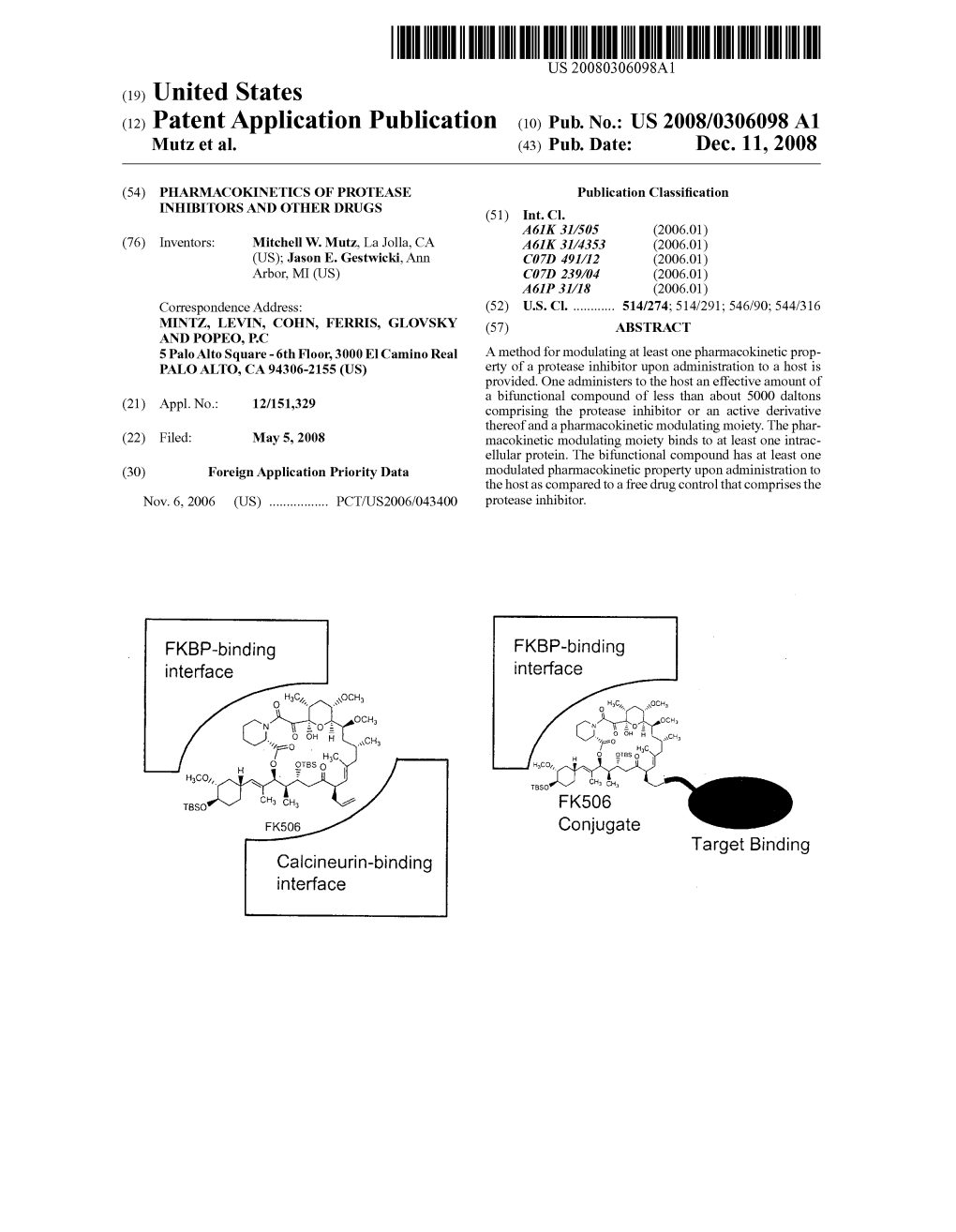
(12) Patent Application Publication (10) Pub. No.: US 2008/0306098 A1 Mutz Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Article PDF/Slides
New Antiretrovirals in Development: Reprinted from The PRN Notebook,™ june 2002. Dr. James F. Braun, Editor-in-Chief. Tim Horn, Executive Editor. Published in New York City by the Physicians’ Research Network, Inc.,® John Graham Brown, Executive Director. For further information and other articles The View in 2002 available online, visit http://www.PRN.org All rights reserved. © june 2002. Roy “Trip” Gulick, md, mph Associate Professor of Medicine, Weill Medical College of Cornell University Director, Cornell Clinical Trials Unit, New York, New York Summary by Tim Horn Edited by Scott Hammer, md espite the fact that 16 antiretro- tiviral activity of emtricitabine was estab- Preliminary results from two random- virals are approved for use in the lished, with total daily doses of 200 mg or ized studies—FTC-302 and FTC-303—were United States, there is an indis- more producing the greatest median viral reported by Dr. Charles van der Horst and putable need for new anti-hiv com- load suppression: 1.72-1.92 log. Based on his colleagues at the 8th croi, held in Feb- pounds that have potent and these data, a once-daily dose of 200 mg ruary 2001 in Chicago (van der Horst, durable efficacy profiles, unique re- was selected for further long-term clinical 2001). FTC-302 was a blinded comparison sistance patterns, patient-friendly dosing study. “This is what we’re looking forward of emtricitabine and lamivudine, both in schedules, and minimal toxicities. To pro- to with emtricitabine,” commented Dr. combination with stavudine (Zerit) and vide prn with a glimpse of drugs current- Gulick. -

( 12 ) United States Patent
US010385067B2 (12 ) United States Patent ( 10 ) Patent No. : US 10 , 385, 067 B2 Carra et al. (45 ) Date of Patent: Aug. 20 , 2019 (54 ) SODIUM (2R , 55 , 13AR ) - 7 , 9 -DIOX0 - 10 ( 56 ) References Cited ( 2 , 4 ,6 - TRIFLUOROBENZYL )CARBAMOYL ) 2 , 3 , 4 , 5 , 7 , 9 , 13 , 13A -OCTAHYDRO - 2 , 5 U . S . PATENT DOCUMENTS METHANOPYRIDO [ 1 ' , 2 ' : 4 , 5 ]PYRAZINO 5 , 814 ,639 A 9 / 1998 Liotta et al . [ 2 , 1 - B ] [ 1 , 3 ]OXAZEPIN - 8 - OLATE 5 , 914 , 331 A 6 / 1999 Liotta et al . 5 ,922 ,695 A 7 / 1999 Arimilli et al . 5 , 935 , 946 A 8 / 1999 Munger, Jr . et al. (71 ) Applicant: Gilead Sciences , Inc ., Foster Ctiy , CA 5 , 977 , 089 A 11/ 1999 Arimilli et al. (US ) 6 ,043 , 230 A 3 / 2000 Arimilli et al. 6 ,620 , 841 B1 9 / 2003 Fujishita et al . (72 ) Inventors : Ernest A . Carra , Foster City , CA ( US ) ; 6 ,642 , 245 B1 11/ 2003 Liotta et al. 6 , 703 , 396 B1 3 / 2004 Liotta et al . Irene Chen , San Mateo , CA (US ) ; 7 , 176 , 220 B2 2 /2007 Satoh et al. Vahid Zia , Palo Alto , CA (US ) 7 ,419 , 969 B2 9 / 2008 Naidu et al. 7 , 550 , 463 B2 6 / 2009 Yoshida (73 ) Assignee : Gilead Sciences , Inc. , Foster City , CA 7 ,635 , 704 B2 12 /2009 Satoh et al. 7 , 858 , 788 B2 12 / 2010 Yoshida et al . (US ) 8 , 129 , 385 B2 3 / 2012 Johns et al . 8 , 148 , 374 B2 4 / 2012 Desai et al. ( * ) Notice : Subject to any disclaimer , the term of this 8 , 188 , 271 B2 5 / 2012 Yoshida et al . -

Modifications to the Harmonized Tariff Schedule of the United States To
U.S. International Trade Commission COMMISSIONERS Shara L. Aranoff, Chairman Daniel R. Pearson, Vice Chairman Deanna Tanner Okun Charlotte R. Lane Irving A. Williamson Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement the Dominican Republic- Central America-United States Free Trade Agreement With Respect to Costa Rica Publication 4038 December 2008 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 18, 2008, set forth in the Appendix hereto, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement the Dominican Republic- Central America-United States Free Trade Agreement, as approved in the Dominican Republic-Central America- United States Free Trade Agreement Implementation Act, with respect to Costa Rica. (This page is intentionally blank) Annex I Effective with respect to goods that are entered, or withdrawn from warehouse for consumption, on or after January 1, 2009, the Harmonized Tariff Schedule of the United States (HTS) is modified as provided herein, with bracketed matter included to assist in the understanding of proclaimed modifications. The following supersedes matter now in the HTS. (1). General note 4 is modified as follows: (a). by deleting from subdivision (a) the following country from the enumeration of independent beneficiary developing countries: Costa Rica (b). -

This Project Has Been Supported with Unrestriced Grants from Abbvie Gilead Sciences HEXAL Janssen-Cilag MSD Viiv Healthcare By
This project has been supported with unrestriced grants from AbbVie Gilead Sciences HEXAL Janssen-Cilag MSD ViiV Healthcare By Marcus Altfeld, Hamburg/Boston (USA) Achim Barmeyer, Dortmund Georg Behrens, Hannover Dirk Berzow, Hamburg Christoph Boesecke, Bonn Patrick Braun, Aachen Thomas Buhk, Hamburg Rob Camp, Barcelona (Spain/USA) Rika Draenert, Munich Christian Eggers, Linz (Austria) Stefan Esser, Essen Gerd Fätkenheuer, Cologne Gunar Günther, Windhoek (Namibia) Thomas Harrer, Erlangen Christian Herzmann, Borstel Christian Hoffmann, Hamburg Heinz-August Horst, Kiel Martin Hower, Dortmund Christoph Lange, Borstel Thore Lorenzen, Hamburg Tim Niehues, Krefeld Christian Noah, Hamburg Ramona Pauli, Munich Ansgar Rieke, Koblenz Jürgen Kurt Rockstroh, Bonn Thorsten Rosenkranz, Hamburg Bernhard Schaaf, Dortmund Ulrike Sonnenberg-Schwan, Munich Christoph D. Spinner, Munich Thomas Splettstoesser (Figures), Berlin Matthias Stoll, Hannover Hendrik Streeck, Essen/Boston (USA) Jan Thoden, Freiburg Markus Unnewehr, Dortmund Mechthild Vocks-Hauck, Berlin Jan-Christian Wasmuth, Bonn Michael Weigel, Schweinfurt Thomas Weitzel, Santiago (Chile) Eva Wolf, Munich HIV 2015/16 www.hivbook.com Edited by Christian Hoffmann and Jürgen K. Rockstroh Medizin Fokus Verlag IV Christian Hoffmann, M.D., Ph.D. ICH Stadtmitte (Infektionsmedizinisches Centrum Hamburg) Glockengiesserwall 1 20095 Hamburg, Germany Phone: + 49 40 2800 4200 Fax: + 49 40 2800 42020 [email protected] Jürgen K. Rockstroh, M.D., Ph.D. Department of Medicine I University of Bonn Sigmund-Freud-Strasse 25 53105 Bonn, Germany Phone: + 49 228 287 6558 Fax: + 49 228 287 5034 [email protected] HIV Medicine is an ever-changing field. The editors and authors of HIV 2015/16 have made every effort to provide information that is accurate and complete as of the date of publication. -

HIV Medicine 2003
Contributing Authors Marcus Altfeld – Boston Georg Behrens – Melbourne Mario Ostrowski – Toronto Andrea Rubbert – Köln Christiane Schieferstein – Frankfurt Reinhold E. Schmidt – Hannover Bruce D. Walker – Boston Eva Wolf – München HIV Medicine 2003 www.HIVMedicine.com Edited by Christian Hoffmann and Bernd Sebastian Kamps Flying Publisher 3 Editors Christian Hoffmann, M.D. University of Schleswig Holstein Infectious Diseases Outpatient Clinic Kiel Chemnitzstr. 33 24116 Kiel, Germany Fax: + 49 431 1697 1273 www.HIVMedicine.com www.SARSReference.com Bernd Sebastian Kamps, M.D. Flying Publisher Rue Saulnier 75009 Paris France www.FlyingPublisher.com HIV Medicine is an ever-changing field. The editors and authors of HIV Medicine 2003 have made every effort to provide information that is accurate and complete as of the date of publication. However, in view of the rapid changes occurring in medical science, HIV prevention and policy, as well as the possibility of human error, this site may contain technical inaccuracies, typographical or other errors. Readers are advised to check the product information currently provided by the manufacturer of each drug to be administered to verify the recommended dose, the method and duration of administration, and contraindications. It is the responsibility of the treating physician who relies on experience and knowledge about the patient to determine dosages and the best treatment for the patient. The information contained herein is provided "as is" and without warranty of any kind. The contributors to this site, including Flying Publisher and AmedeoGroup, disclaim responsibility for any errors or omissions or for results obtained from the use of information contained herein. © 2003 by Flying Publisher – Paris, Cagliari, Wuppertal, Sevilla Assistant Editors: Nyasha Bakare, Dianne Lydtin Design: Attilio Baghino, www.a4w.it ISBN: 3-924774-37-4 4 Preface Hardly any field of medicine has ever undergone a similar stormy development to that of the therapy of HIV infection. -

Downloaded In.Sdf Format from the Pubchem Database (
In silico Prediction of Mozenavir as potential drug for SARS-CoV-2 infection via Binding Multiple Drug Targets Estari Mamidalaa*, Rakesh Davellab, Swapna Gurrapuc, Munipally Praveen Kumard, Abhiave a,b,c,d Infectious Diseases Research Lab, Department of Zoology, Kakatiya University, Warangal, Telangana-506 009, India e Division of ISRM, Indian Council of Medical Research (ICMR), Department of Health Research, New Delhi, India *Corresponding should be addressed to Estari Mamidala: [email protected] Keywords: Mozenavir, SARS-CoV-2, Mpro, ACE-2, RdRp, S glycoprotein, Furin ABSTRACT Since the epidemic began in November 2019, no viable medicine against SARS-CoV-2 has been discovered. The typical medication discovery strategy requires several years of rigorous research and development as well as a significant financial commitment, which is not feasible in the face of the current epidemic. Through molecular docking and dynamic simulation studies, we used the FDA-approved drug mezonavir against the most important viral targets, including spike (S) glycoprotein, Transmembrane serine protease 2 (TMPRSS2), RNA-dependent RNA polymerase (RdRp), Main protease (Mpro), human angiotensin-converting enzyme 2 (ACE-2), and furin. These targets are critical for viral replication and infection propagation because they play a key role in replication/transcription and host cell recognition. Molecular docking revealed that the antiviral medication mozenavir showed a stronger affinity for SARS-CoV-2 target proteins than reference medicines in this investigation. We discovered that mozenavir increases the complex's stability and validates the molecular docking findings using molecular dynamics modelling. Furin, a target protein of COVID-19, has a greater binding affinity (-12.04 kcal/mol) than other COVID-19 target proteins, forming different hydrogen bonds and polar and hydrophobic interactions, suggesting that it might be used as an antiviral treatment against SARS- CoV-2. -

Current and Novel Inhibitors of HIV Protease
Viruses 2009, 1, 1209-1239; doi:10.3390/v1031209 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Current and Novel Inhibitors of HIV Protease Jana Pokorná 1,5, Ladislav Machala 2,3, Pavlína Řezáčová 1,4 and Jan Konvalinka 1,4,5,* 1 Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Gilead Sciences and IOCB Research Center, Flemingovo n. 2, 166 10, Prague 6, Czech Republic; E-Mails: [email protected] (J.P.); [email protected] (P.R.) 2 AIDS Center, Department of Infectious Diseases, University Hospital Bulovka, Budínova 2, 120 00, Prague 8, Czech Republic; E-Mail: [email protected] 3 Department of Infectious Diseases, Third Faculty of Medicine, Charles University in Prague, Prague, Czech Republic 4 Institute of Molecular Genetics, Academy of Sciences of the Czech Republic, Flemingovo n. 2, 166 10, Prague 6, Czech Republic 5 Department of Biochemistry, Faculty of Science, Charles University in Prague, Hlavova 8, 128 43, Prague 2, Czech Republic * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +420-220-183-218; Fax: +420-220-183-378. Received: 8 October 2009; in revised form: 7 December 2009 / Accepted: 7 December 2009 / Published: 11 December 2009 Abstract: The design, development and clinical success of HIV protease inhibitors represent one of the most remarkable achievements of molecular medicine. This review describes all nine currently available FDA-approved protease inhibitors, discusses their pharmacokinetic properties, off-target activities, side-effects, and resistance profiles. The compounds in the various stages of clinical development are also introduced, as well as alternative approaches, aiming at other functional domains of HIV PR. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic -

Antiviral Drugs in the Treatment of Aids: What Is in the Pipeline ?
October 15, 2007 EU RO PE AN JOUR NAL OF MED I CAL RE SEARCH 483 Eur J Med Res (2007) 12: 483-495 © I. Holzapfel Publishers 2007 ANTIVIRAL DRUGS IN THE TREATMENT OF AIDS: WHAT IS IN THE PIPELINE ? Hans-Jürgen Stellbrink Infektionsmedizinisches Centrum Hamburg ICH, Hamburg, Germany Abstract even targeting immune responses have to be devel- Drug development in the field of HIV treatment is oped. Continued drug development serves the ulti- rapid. New nucleoside analogues (NRTI), non-nucleo- mate goal of normalization of life expectancy. side analogue reverse transcriptase inhibitors (NNR- TI), and protease inhibitors (PI) are currently being in- METHODS vestigated in human trials. Furthermore, inhibitors of HIV attachment, fusion and integrase with novel Drug development in the field of HIV infection is modes of action are being developed, which offer new highly competitive, and a company’s decision to pur- perspectives for the goal of a normalization of life-ex- sue or discontinue the development of a drug is dri- pectancy in HIV-infected individuals. The most ad- ven by economic rather than scientific considerations. vanced compounds likely to become licensed soon in- Of all candidate compounds, only a few reach the lev- clude the NNRTIs rilpivirine and etravirine, the inte- el of trials in humans, and some exhibit lack of effica- grase inhibitors raltegravir and elvitegravir, and mar- cy or toxicity problems at this stage. Some compounds aviroc and vicriviroc, novel inhibitors of the CCR5 also have no obvious advantage over currently avail- chemokine receptor, which functions as the major able ones, so that their development is discontinued. -

Shudda, Cudda, Wudda. Reevaluating the Treatment
Drugs in Development: Going, Coming, Gone line Clinical Myopia at NIAID From the Treatment Action Group (TAG): Dátà Vu at Summer IAS Meeting A monthly paper of research and policy TAGline en español Volume 8 Issue 7 September 2001 Shreds of Evidence Down the Pike Investigational New Drugs Currently Shudda, Cudda, Wudda. Being Tested in Humans, by Class Argentina IAS Reevaluating the Nucleoside RTI 3 Snoozer Prompts Non-nucleoside RTI 7 Treatment Revolution Protease inhibitor 4 Systematic Review of After the Fall Fusion/binding inhibitor 5 Drug Pipeline Cellular factors inhibitor 4 Other 2 ‘Six years of lost data’ Total 25 ‘Underwhelmingly innovative’ David Barr revisits the changes to the The International AIDS Society inau- federal when-to-start recommendations Economía de Drogas gurated its new program of summer announced earlier this year and urges research meetings with a sleeper of a us to consider both the roots and the Renegado Antiretroviral gathering in the southern hemisphere ramifications of this extraordinary devel- Lucha Contra La Moda port city of Buenos Aires. Mark opment. While acknowleding the Con Dúo Didanosina, Harrington attended on behalf of TAG predicament of a Monday morning and sifted the new from the old to pre- quarterback, his words are frank and Apuestando Su Futuro pare this report for TAGline. hard-hitting. “How many people have En Barreras Genéticas only suffered from the side effects of t midwinter in Buenos Aires the * * * treatment—and not from HIV infec- Adays are short and the weather is tion?” he asks. And “where is the ‘Monoterapia enforzada’ cool, like summer in San Francisco. -

Hoffmann Rockstroh |
Hoffmann| Rockstroh HIV 2015/2016 www.hivbook.com Medizin Fokus Verlag This project has been supported with unrestriced grants from AbbVie Gilead Sciences HEXAL Janssen-Cilag MSD ViiV Healthcare By Marcus Altfeld, Hamburg/Boston (USA) Achim Barmeyer, Dortmund Georg Behrens, Hannover Dirk Berzow, Hamburg Christoph Boesecke, Bonn Patrick Braun, Aachen Thomas Buhk, Hamburg Rob Camp, Barcelona (Spain/USA) Rika Draenert, Munich Christian Eggers, Linz (Austria) Stefan Esser, Essen Gerd Fätkenheuer, Cologne Gunar Günther, Windhoek (Namibia) Thomas Harrer, Erlangen Christian Herzmann, Borstel Christian Hoffmann, Hamburg Heinz-August Horst, Kiel Martin Hower, Dortmund Christoph Lange, Borstel Thore Lorenzen, Hamburg Tim Niehues, Krefeld Christian Noah, Hamburg Ramona Pauli, Munich Ansgar Rieke, Koblenz Jürgen Kurt Rockstroh, Bonn Thorsten Rosenkranz, Hamburg Bernhard Schaaf, Dortmund Ulrike Sonnenberg-Schwan, Munich Christoph D. Spinner, Munich Thomas Splettstoesser (Figures), Berlin Matthias Stoll, Hannover Hendrik Streeck, Essen/Boston (USA) Jan Thoden, Freiburg Markus Unnewehr, Dortmund Mechthild Vocks-Hauck, Berlin Jan-Christian Wasmuth, Bonn Michael Weigel, Schweinfurt Thomas Weitzel, Santiago (Chile) Eva Wolf, Munich HIV 2015/16 www.hivbook.com Edited by Christian Hoffmann and Jürgen K. Rockstroh Medizin Fokus Verlag IV Christian Hoffmann, M.D., Ph.D. ICH Stadtmitte (Infektionsmedizinisches Centrum Hamburg) Glockengiesserwall 1 20095 Hamburg, Germany Phone: + 49 40 2800 4200 Fax: + 49 40 2800 42020 [email protected] Jürgen K. Rockstroh, M.D., Ph.D. Department of Medicine I University of Bonn Sigmund-Freud-Strasse 25 53105 Bonn, Germany Phone: + 49 228 287 6558 Fax: + 49 228 287 5034 [email protected] HIV Medicine is an ever-changing field. The editors and authors of HIV 2015/16 have made every effort to provide information that is accurate and complete as of the date of publication.