Pollution in People © 2006 Toxic-Free Legacy Coalition Printed on 100% Post-Consumer Content Recycled Paper, Not Rebleached with Chlorine Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Newsletter Term 1 19-20
Heritage Skills Event Fifteen of our students attended a workshop session in Perth City Centre aimed at promoting careers using traditional building skills. The event, organised by Perth and Kinross Heritage Trust, gave the pupils an experience of using traditional skills of carpentry, stone masonry, slating and painting. Our students thoroughly enjoyed the event, and some of them highly impressed the organisers! The event was even attended by Depute First Minister, John Swinney MSP, who took time out of his busy schedule to chat to our students and congratulate them for taking part. Primary Language Ambassadors Welcome to our new group of P5-7 Language Ambassadors for this year! They are enthusiastic about celebrating all the languages spoken in St John's Academy and are looking forward to supporting language learning throughout the school. This year's Language Ambassadors are already bursting with ideas, including competitions, lunch clubs and quizzes. Their first task has been to celebrate 'European Day of Languages' on Thursday, 26th September. European Day of Languages On Thursday, 26th September, we celebrated the European Day of Languages. Many classes took part in numerous activities for the promotion of languages. We had fun dressing up in costumes from around the world, completed quizzes about Europe, translated Follow us on Twitter music titles and increased our vocabulary for @sja_languages greeting people in a language other than their mother tongue as well as discussing the benefits of having knowledge of more than one language. Our pupils approached the many activities with great gusto! In addition, on a recent visit to the school, Bishop Stephen Robson was more than happy to help fly the language flag by joining the Senior Leadership Team for a photograph to celebrate this special day. -

Review of Exposure Data and Assessments for Select Dialkyl
TABLE OF CONTENTS 1. INTRODUCTION ........................................................................................................... 1-1 2. PRODUCTION AND USE OF PHTHALATES ............................................................. 2-1 2.1. PRODUCTION OF PHTHALATES ................................................................... 2-1 2.1.1. Production of DEHP ................................................................................ 2-1 2.1.2. Production of DBP ................................................................................... 2-2 2.1.3. Production of BBP ................................................................................... 2-2 2.1.4. Production of DINP ................................................................................. 2-3 2.1.5. Production of DIDP ................................................................................. 2-3 2.1.6. Production of DnOP ................................................................................. 2-4 2.2. USE OF PHTHALATES ..................................................................................... 2-4 3. PHYSICO-CHEMICAL AND ENVRONMENTAL FATE PROPERTIES OF PHTHALATES ................................................................................. 3-1 3.1. PHYSICO-CHEMICAL PROPERTIES OF PHTHALATES ............................. 3-1 3.1.1. Benzyl butyl phthalate (BBP) .................................................................. 3-1 3.1.2. Di-n-butyl phthalate (DBP) ..................................................................... -

Polyvinyl Chloride - Wikipedia Polyvinyl Chloride
1/24/2020 Polyvinyl chloride - Wikipedia Polyvinyl chloride Polyvinyl chloride (/ˌpɒlivaɪnəl ˈklɔːraɪd/;[5] colloquial: Polyvinyl chloride polyvinyl, vinyl;[6] abbreviated: PVC) is the world's third-most widely produced synthetic plastic polymer, after polyethylene and polypropylene.[7] About 40 million tonnes are produced per year. PVC comes in two basic forms: rigid (sometimes abbreviated as RPVC) and flexible. The rigid form of PVC is used in construction for pipe and in profile applications such as doors and windows. It is also used in making bottles, non-food packaging, food-covering sheets,[8] and cards (such as bank or membership cards). It can be made softer and more flexible by the addition of plasticizers, the most widely used being phthalates. In this form, it is also used in plumbing, electrical cable insulation, imitation leather, flooring, signage, phonograph records,[9] inflatable products, and many applications where it replaces rubber.[10] With cotton or linen, it is used to make canvas. Pure polyvinyl chloride is a white, brittle solid. It is insoluble in alcohol but slightly soluble in tetrahydrofuran. Contents Discovery Production Microstructure Names Producers IUPAC name Additives poly(1-chloroethylene)[1] Phthalate plasticizers Di-2ethylhexylphthalate Other names Metal stabilizers Polychloroethylene Heat stabilizers Identifiers Properties CAS Number 9002-86-2 (http://ww Mechanical w.commonchemistry. Thermal and fire org/ChemicalDetail.a Electrical spx?ref=9002-86-2) Chemical Abbreviations PVC Applications ChEBI CHEBI:53243 -

Effects on Bromine, Chlorine and SO2 Under Air Firing and Oxy-Fuel
GAS-PHASE MERCURY OXIDATION: EFFECTS OF BROMINE, CHLORINE AND SO2 UNDER AIR FIRING AND OXY-FUEL CONDITIONS, EXPERIMENTAL AND MODELING STUDY by Paula Andrea Buitrago A dissertation submitted to the faculty of The University of Utah in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Chemical Engineering The University of Utah August 2011 Copyright © Paula Andrea Buitrago 2011 All Rights Reserved STATEMENT OF DISSERTATION APPROVAL The dissertation of Paula Andrea Bnitrago has been approved by the following supervisory committee members: Geoffrey D. Silcox , Chair 4/1112011 Date Approved JoAnn S. Lighty , Member 4/1112011 Date Approved Jost Wendt , Member 4/18/2011 bate Approved Connie Senior , Member 4/15/2011 Date Approved Eric G. Eddings , Member 4/1112011 bate Approved and by JoAnn S. Lighty , Chair of ----------------~~~~==~~--------------- the Department of Chemical Engineering and by Charles A. Wight, Dean of The Graduate School. ABSTRACT The mercury in coal is emitted in its elemental state when the coal is burned. As the combustion flue gas cools, reactions under homogeneous and heterogeneous conditions between mercury and species such chlorine, bromine, SOx and NOx can take place. The temperature and concentration of these species determines the extent of mercury oxidation. The main objective of this study was to evaluate the effects of bromine, chlorine, SOx and NOx on gas-phase mercury oxidation reactions in flue gas. This study used a methane-fired, bench-scale reactor and CHEMKIN software for performing kinetic calculations. The model included reaction pathways to account for halogen, mercury, and combustion chemistry. The experimental results showed that chlorine is not effective as a gas-phase oxidant of mercury compared with other halogens such as bromine. -

Ross Bennett @Golder.Com
REPORT ON-PROPERTY SITE INVESTIGATION WORK PLAN Saint-Gobain Performance Plastics Facility 701 Daniel Webster Highway in Merrimack, New Hampshire Submitted to: New Hampshire Department of Environmental Services Hazardous Waste Remediation Bureau 29 Hazen Drive, PO Box 95 Concord, New Hampshire 03302 Submitted by: Saint-Gobain Performance Plastics Corp. 14 McCaffrey Street Hoosick Falls, New York 12090 Submitted by: Golder Associates Inc. 670 North Commercial Street, Suite 103 Manchester, New Hampshire, USA 03101 June 8, 2018 June 8, 2018 1668623 Table of Contents 1.0 INTRODUCTION ............................................................................................................................................. 1 2.0 BACKGROUND .............................................................................................................................................. 1 2.1 Property and Facility History ................................................................................................................ 1 2.2 Summary of Previous Investigations .................................................................................................... 3 3.0 PRELIMINARY CONCEPTUAL SITE MODEL .............................................................................................. 3 3.1 Geologic and Hydrogeologic Setting .................................................................................................... 4 3.2 Potential Releases and Transport Mechanisms ................................................................................. -

Adapting at the Speed of Style with HP Server and Storage Blades
Adapting at the speed of style with HP server and storage blades Speeding electronic orders by sixfold while offering 99.99% uptime with HP servers and storage “Before, it could take up to a half hour to process a large EDI. Now with a clustered Oracle environment on our HP BL460c server blades, those orders are processed sixfold faster in under five minutes.” — Enrico Bozzetti, IT manager, Excelled Sheepskin & Leather Objective Eliminate ordering bottlenecks while boosting availability to enable growth Approach Create a clustered environment for Oracle Database 10g R2 using HP Converged Infrastructure technologies including ProLiant G6 server blades and HP StorageWorks storage blades in a single HP BladeSystem c3000 enclosure HP customer case study: HP ProLiant IT improvements BL460c G6 server blades • Sixfold faster electronic data interchange (EDI) HP StorageWorks storage processing blades • 99.99% uptime, with 60–120 hours of planned Industry: manufacturing downtime eliminated • Saving 40 hours per month through remote Fashion forward server management Wandering the clothing aisles at a major department • 60% faster storage provisioning store can be like an adventure through your various personality traits. Do I go conservative and safe, Business benefits or am I in the mood for an of-the-moment fashion • Eliminates electronic order bottlenecks statement? A good way to do both is by drifting over to the leather goods section, where the sleek and • Faster report generation supple jackets are timelessly cool. • 20% year-over-year data growth supported by an IT staff of two Though department stores do a great job of merchandising and marketing the end product, • Near 60% projected savings on power and creating these works of self expression is left to cooling in five years vs. -

PVC Jars and Bottles
PVC Jars and Bottles Polyvinyl chloride, (IUPAC Poly(chloroethanediyl)) commonly abbreviated PVC, is a thermoplastic polymer. It is a vinyl polymer constructed of repeating vinyl groups (ethenyls) having one of their hydrogens replaced with a chloride group. Polyvinyl chloride is the third most widely produced plastic, after polyethylene and polypropylene.PVC is widely used in construction because it is cheap, durable, and easy to assemble. PVC production is expected to exceed 40 million tons by 2016. It can be made softer and more flexible by the addition of plasticizers, the most widely used being phthalates. In this form, it is used in clothing and upholstery, and to make flexible hoses and tubing, flooring, to roofing membranes, and electrical cable insulation. It is also commonly used in figurines and in inflatable products such as waterbeds, pool toys, and inflatable structures. Preparation Polyvinyl chloride is produced by polymerization of the vinyl chloride monomer (VCM), as shown.Since about 57% of its mass is chlorine, creating a given mass of PVC requires less petroleum than many other polymers.[citation needed] However, because PVC also has a much higher density than hydrocarbon polymers, and chlorine production has its own energy requirements, this ends up being of little practical relevance in the production of most solid objects. By far the most widely used production process is suspension polymerization. In this process, VCM and water are introduced into the polymerization reactor and a polymerization initiator, along with other chemical additives, are added to initiate the polymerization reaction. The contents of the reaction vessel are continually mixed to maintain the suspension and ensure a uniform particle size of the PVC resin. -

EFGS-SOP-137-R02 and CV-AFS (EPA Method 1631, Rev E)
Document Title: Eurofins Document Reference: Mercury in Water by Oxidation, Purge & Trap EFGS-SOP-137-R02 and CV-AFS (EPA Method 1631, Rev E) Eurofins Document Reference EFGS-SOP-137-R02 Revision 2 Effective Date 6/17/2013 Status Final Historical/Local Document Number FGS-SOP-137.02 Local Document Level Level 3 Local Document Type SOP Local Document Category NA Prepared by Ryan Nelson Reviewed Dave Wunderlich and Patrick Garcia-Strickland and Approved by Revision: 2 Effective Date: 6/17/2013 Page 1 of 20 COMPANY CONFIDENTIAL. All Eurofins Frontier Global Sciences standard operating procedures contain proprietary information and are protected by Washington State Law. Proprietary information must be maintained with the strictest of confidence and must not be used or appropriated to benefit any party without prior written consent from Eurofins Frontier Global Sciences. Document Title: Eurofins Document Reference: Mercury in Water by Oxidation, Purge & Trap EFGS-SOP-137-R02 and CV-AFS (EPA Method 1631, Rev E) Table of Contents 1 Revision Log: ......................................................................................................................... 4 2 Reference: ............................................................................................................................. 4 3 Cross Reference: ................................................................................................................... 5 4 Purpose: ............................................................................................................................... -

School Uniform St John’S Rc Academy
OUR AIMS & VALUES TREE SCHOOL UNIFORM ST JOHN’S RC ACADEMY ST JOHN’S RC ACADEMY ST JOHN’S RC ACADEMY North Inch Community Campus Gowans Terrace Perth PH1 5BF Phone: 01738 454300 MAKING OUR UNIFORM UNIFORM E-mail: [email protected] Twitter: @st_johnsacademy May 2015 OUR UNIFORM In St John’s RC Academy we ‘Seek the PRIMARY UNIFORM SECONDARY UNIFORM Good’ in ourselves, in others and in every aspect of our lives. Black dress trousers/shorts or skirt/culottes Black dress trousers or skirt We are proud to be part of our commu- nity and we have agreed a uniform White school shirt White school shirt which we are proud to wear. Grey jumper/cardigan (with or without the school badge) S1-3: Black jumper/cardigan/hoodie (with or without the We recognise that through wearing our which allows the school tie to be seen school badge) which allows the school tie to be seen uniform we are demonstrating that we School tie School tie are ready to work and ready to learn. BGE (for P1-S3) tie available at the school office BGE tie (for P1-S3); Senior tie (for S4-6) available at school office We also believe that our uniform helps Black shoes/trainers with black laces Black shoes/trainers with black laces everyone to belong—it is an important Plain black only Plain black only symbol that we are a community and Purple and white summer dress during the summer School blazer with purple braiding that each of us contributes to making months (optional) S1-3: Optional. -
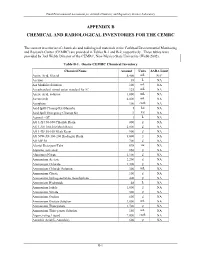
Appendix B Chemical and Radiological Inventories for the Cemrc
Final Environmental Assessment for Actinide Chemistry and Repository Science Laboratory APPENDIX B CHEMICAL AND RADIOLOGICAL INVENTORIES FOR THE CEMRC The current inventories of chemicals and radiological materials at the Carlsbad Environmental Monitoring and Research Center (CEMRC) are provided in Tables B-1 and B-2, respectively. These tables were provided by Joel Webb, Director of the CEMRC, New Mexico State University (Webb 2002). Table B-1. Onsite CEMRC Chemical Inventory Chemical Name Amount Units SARA Limit Acetic Acid, Glacial 5,400 mL NAa Acetone 38 L NA AA Modifier Solution 100 mL NA AccuStandard mixed anion standard for IC 125 mL NA Acetic Acid, solution 1,000 mL NA Acetonitrile 4,000 mL NA Acetylene 100 cu.ft. NA Acid Spill Cleanup Kit (Hazorb) 1 kit NA Acid Spill Emergency Cleanup Kit 3 kit NA Aerosol - OT 1 L NA AG 1-X4 50-100 Chloride Resin 800 g NA AG 1-X8 100-200 Mesh Resin 2,000 g NA AG 1-X8 50-100 Mesh Resin 900 g NA AG 50W-X8 100-200 Hydrogen Resin 3,000 g NA AG MP 50 700 g NA Alcojet Detergent/Tabs 698 oz NA Alumina, activated 850 g NA Aluminum Nitrate 2,100 g NA Ammonium Acetate 2,200 g NA Ammonium Chloride 1,300 g NA Ammonium Chloride Solution 300 mL NA Ammonium Citrate 100 g NA Ammonium hydrogenoxalate, hemihydrate 400 g NA Ammonium Hydroxide 48 L NA Ammonium Iodide 1,000 g NA Ammonium Nitrate 500 g NA Ammonium Oxalate 600 g NA Ammonium Oxalate Solution 2,000 mL NA Ammonium Thiocyanate 1,700 g NA Ammonium Thiocyanate Solution 150 mL NA Argon, refrig. -

Aggregate Exposures to Phthalates in Humans
Aggregate Exposures to Phthalates in Humans July 2002 ACKNOWLEDGMENTS Contributors Joseph DiGangi, PhD, USA, Ted Schettler MD, MPH, USA Madeleine Cobbing, UK Mark Rossi, MA, USA Reviewers HCWH thanks the following individuals for reviewing an earlier draft of this report. Their comments and sug- gestions were invaluable and substantially improved the manuscript. We are grateful for their contribution. Their review, however, does not constitute endorsement of the report or its conclusions. Two additional reviewers chose to remain anonymous. Earl Gray PhD Michael McCally MD, PhD The contributing authors would also like to thank Cecilia DeLoach, Tracey Easthope, Per Rosander, Jamie Harvie and Charlotte Brody for their editing and proofreading of this report. Health Care Without Harm 1755 S St. NW, Suite 6B • Washington, DC 20009 • www.noharm.org • 202-234-0091 Contents Acknowledgements......................................................................................ii Executive Summary.....................................................................................1 Abbreviations..............................................................................................4 Preface......................................................................................................5 Introduction................................................................................................6 Phthalates in Consumer Products.................................................................9 Phthalate Toxicity ......................................................................................14 -

Material Safety Data Sheet
MATERIAL SAFETY DATA SHEET Trade Name: Power Capsule, #8791 Date Prepared: August 1999 Revised: March 2004 Section I: - Source Information Manufacturer's Name: Michigan State Industries, Inc. Address: Ryan Correctional Facility Emergency Phone: (313) 368-3200 x51412 (800) 222-1222 Poison Control Technical Information: (313) 368-3200 x51412 Chemical Name: Detergent Section II - Hazardous Ingredients/Identify Information Hazardous Components CAS No. OSHA PEL* ACGIH TLV* Percentage Sodium Hydroxide 1310-73-2 2mg/m3 2mg/m3 <22% Sodium Metasilicate 6834-92-0 <9% No exposure limits have been established for this specific product however, the following limits may apply Sodium Dichloro-S-Triazinetrione 2893-78-9 10mg/m3 <3% Although OSHA and ACGIH have not established specific exposure limits for this material, they have established for the following nuisance dusts. OSHA PEL/TWA: TOTAL 15mg/m3; RESPIRABLE 5mg/m3 ACGIH TLV/TWA TOTAL 10mg/m3; RESPIRABLE 5mg/m3 *Exposure limits expressed as eight-hour time-weighted average concentrations in parts of contaminant per million parts of air by volume (ppm). "Other Limits" are recommended by the National Institute for Occupational Safety and Health (NIOSH) and are time-weighted over ten hours unless otherwise specified. Section III - Physical/Chemical Characteristics Boiling Point: N/A Specific Gravity: NO DATA Vapor Pressure: N/A Vapor Density: N/A Evaporation Rate: (Ethyl Ether =1): N/A Solubility in Water: Complete Appearance and Color: White powder, chlorine odor Section IV- Fire and Explosion Hazard Data: Flash Point: N/A Flammable Limits: LEL: N/A UEL: N/A Extinguishing Media: This material is not combustible. Foam, carbon dioxide or dry chemical may be used in areas where this product is stored.