Bile Pigment Excretion: a Comparison of the Biliary Excretion of Bilirubin and Bilirubin Derivatives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urobiliverdin, a New Bile Pigment Deriving from Uroporphyrin Eva Benedikt and Hans-Peter Köst Botanisches Institut, Universität München, Menzingerstr
Urobiliverdin, a New Bile Pigment Deriving from Uroporphyrin Eva Benedikt and Hans-Peter Köst Botanisches Institut, Universität München, Menzingerstr. 67, D-8000 München 19, Bundesrepublik Deutschland Z. Naturforsch. 38 c, 753-757 (1983); received May 4, 1983 Semisynthetic Bile Pigments, Urobiliverdin III, Coprobiliverdin III, Biliverdin IX, Uroporphyrin III 5-Aminolevulinic acid is incubated with a crude enzyme extract from Rhodopseudomonas spheroides, mutant R 26. The formed porphyrins (main product: uroporphyrin III) are isolated. Incorporation of iron, ring-splitting by coupled oxydation and subsequent iron removal leads to a mixture of pigments, from which urobiliverdin, a new bile pigment with eight carboxylic acid side chains, is isolated. It is characterized by its chromatographic behaviour, chromic acid degra dation and UV-vis spectroscopy. Introduction the photosynthetic bacteria, Rhodopseudomonas spheroides, mutant R26 was a gift from Prof. Naturally occurring open chained tetrapyrrolic H. Scheer, Munich. Marker porphyrins were pur systems (bile pigments) usually bear two carboxylic chased from Porphyrin products (Logan, USA). acid side chains, namely propionic acid side chains. Chromic acid degradation was performed accord Examples are phycocyanobilin [1-5], phycoerythro- ing to the procedure developed by Rüdiger [21]; the bilin [5, 6], phytochromobilin [7, 8], the biliar pig degradation products were separated on silicagel 60 ment bilirubin ([9], here older literature) and sterco- coated HPTLC plates (E. Merck, Darmstadt, FRG) bilin [9] which is encountered in the faeces. using the solvent system chloroform-ethyl acetate- All of these pigments probably derive from proto cyclohexane = 6:3:1. The imides formed were heme as has been shown experimentally in a identified by comparison with authentic standards. -

PDF File Includes: Main Text Figures 1 to 4 Supplemental Text Supplemental Figures S1 to S8 Supplemental Tables
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.08.414581; this version posted December 9, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Structural basis of the protochromic green/red photocycle of the chromatic acclimation sensor RcaE Takayuki Nagaea, Masashi Unnob, Taiki Koizumic, Yohei Miyanoirid, Tomotsumi Fujisawab, Kento Masuif, Takanari Kamof, Kei Wadae, Toshihiko Ekif, Yutaka ItoC, Yuu Hirosef and Masaki Mishimac aSynchrotron Radiation Research Center, Nagoya University, Chikusa, Nagoya 464-8603, Japan; bDepartment of Chemistry and Applied Chemistry, Faculty of Science and Engineering, Saga University, Saga 840-8502, Japan; cDepartment of Chemistry, Graduate School of Science, Tokyo Metropolitan University, 1-1 Minamiosawa, Hachioji 192-0397, Japan; dInstitute for Protein Research , Osaka University , 3-2 Yamadaoka , Suita , Osaka 565-0871 , Japan; eDepartment of Medical Sciences, University of Miyazaki, Miyazaki 889-1692, Japan; fDepartment of Environmental and Life Sciences, Toyohashi University of Technology, Toyohashi, Aichi 441-8580, Japan 1To whom correspondence should be addressed 1Masaki Mishima Email: [email protected] 1Yuu Hirose Email: [email protected] 1 Masashi Unno Email: [email protected] Takayuki Nagae: 0000-0001-7016-5183 Tomotsumi Fujisawa: 0000-0002-3282-6814 Masashi Unno: 0000-0002-5016-6274 Yohei Miyanoiri: 0000-0001-6889-5160 Yuu Hirose: 0000-0003-1116-8979 Kei Wada: 0000-0001-7631-4151 Yutaka Ito: 0000-0002-1030-4660 Masaki Mishima: 0000-0001-7626-7287 Classification Biological Sciences Keywords cyanobacteriochrome, phytochrome, bilin, NMR, crystallography Author Contributions MM, YH, and MU designed the research and wrote the manuscript. -

Hyperbilirubinemia
Porphyrins Porphyrins (Porphins) are cyclic tetrapyrol compounds formed by the linkage )). of four pyrrole rings through methenyl bridges (( HC In the reduced porphyrins (Porphyrinogens) the linkage of four pyrrole rings (tetrapyrol) through methylene bridges (( CH2 )) The characteristic property of porphyrins is the formation of complexes with the metal ion bound to nitrogen atoms of the pyrrole rings. e.g. Heme (iron porphyrin). Proteins which contain heme ((hemoproteins)) are widely distributed e.g. Hemoglobin, Myoglobin, Cytochromes, Catalase & Tryptophan pyrrolase. Natural porphyrins have substituent side chains on the eight hydrogen atoms numbered on the pyrrole rings. These side chains are: CH 1-Methyl-group (M)… (( 3 )) 2-Acetate-group (A)… (( CH2COOH )) 3-Propionate-group (P)… (( CH2CH2COOH )) 4-Vinyl-group (V)… (( CH CH2 )) Porphyrins with asymmetric arrangement of the side chains are classified as type III porphyrins while those with symmetric arrangement of the side chains are classified as type I porphyrins. Only types I & III are present in nature & type III series is more important because it includes heme. 1 Heme Biosynthesis Heme biosynthesis occurs through the following steps: 1-The starting reaction is the condensation between succinyl-CoA ((derived from citric acid cycle in the mitochondria)) & glycine, this reaction is a rate limiting reaction in the hepatic heme synthesis, it occurs in the mitochondria & is catalyzed by ALA synthase (Aminolevulinate synthase) enzyme in the presence of pyridoxal phosphate as a cofactor. The product of this reaction is α-amino-β-ketoadipate which is rapidly decarboxylated to form δ-aminolevulinate (ALA). 2-In the cytoplasm condensation reaction between two molecules of ALA is catalyzed by ALA dehydratase enzyme to form two molecules of water & one 2 molecule of porphobilinogen (PBG) which is a precursor of pyrrole. -

Retrograde Bilin Signaling Enables Chlamydomonas Greening and Phototrophic Survival
Retrograde bilin signaling enables Chlamydomonas greening and phototrophic survival Deqiang Duanmua, David Caserob,c, Rachel M. Dentd, Sean Gallahere, Wenqiang Yangf, Nathan C. Rockwella, Shelley S. Martina, Matteo Pellegrinib,c, Krishna K. Niyogid,g,h, Sabeeha S. Merchantb,e, Arthur R. Grossmanf, and J. Clark Lagariasa,1 aDepartment of Molecular and Cellular Biology, University of California, Davis, CA 95616; bInstitute for Genomics and Proteomics, cDepartment of Molecular, Cell and Developmental Biology, and eDepartment of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095; dDepartment of Plant and Microbial Biology, gHoward Hughes Medical Institute, and hPhysical Biosciences Division, Lawrence Berkeley National Laboratory, University of California, Berkeley, CA 94720; and fDepartment of Plant Biology, Carnegie Institution for Science, Stanford, CA 94305 Contributed by J. Clark Lagarias, December 20, 2012 (sent for review October 30, 2012) The maintenance of functional chloroplasts in photosynthetic share similar mechanisms for light sensing and retrograde sig- eukaryotes requires real-time coordination of the nuclear and naling. However, many chlorophyte genomes lack phytochromes plastid genomes. Tetrapyrroles play a significant role in plastid-to- (21–24), a deficiency offset by a larger complement of flavin- and nucleus retrograde signaling in plants to ensure that nuclear gene retinal-based sensors (25–30). expression is attuned to the needs of the chloroplast. Well-known Despite the absence of phytochromes, all known chlorophyte sites of synthesis of chlorophyll for photosynthesis, plant chlor- genomes retain cyanobacterial-derived genes for the two key oplasts also export heme and heme-derived linear tetrapyrroles enzymes required for bilin biosynthesis (Fig. 1A): a heme oxygen- (bilins), two critical metabolites respectively required for essential ase (HMOX1) and a ferredoxin-dependent bilin reductase (PCYA). -

Determination of Urinary Porphyrin by DEAE-Cellulose Chromatography and Visual Spectrophotometry
A n n a l s o f C l i n i c a l a n d L a b o r a t o r y S c i e n c e , Vol. 4, No. 1 Copyright © 1974, Institute for Clinical Science Determination of Urinary Porphyrin by DEAE-Cellulose Chromatography and Visual Spectrophotometry MARTHA I. WALTERS, P h .D.* Ohio Valley Hospital, Steubenville, OH 43952 ABSTRACT A simple, reliable and rapid method for the isolation and determination of urinary porphyrins is described. Both uroporphyrin and coproporphyrin are quantitatively adsorbed by DEAE cellulose. Urobilinogen, urobilin, and bili rubin are removed with sodium acetate. The isolated porphyrins, eluted with 1 N HC1, are measured spectrophotometrically, and the excretion is related to that of creatinine. Porphyrin excretion was studied in normal adults and children, and in patients with disorders of porphyrin metabolism ( e.g., in lead poisoning, hemolytic anemia, hepatic disease and porphyria) in order to eval uate the method. The coefficient of variation of replicate analyses of a sample containing 200 ¡xg porphyrin per gm creatinine was 3.5 percent. The correlation coefficient between 83 duplicate analyses was 0.993. Introduction or adsorption onto an adsorbant followed Patients with porphyria show great in by elution. Methods combining extraction creases in the excretion of uroporphyrin, and adsorption have been the most com coproporphyrin and their precursors indi monly used,3’12 but procedures employing cating a primary abnormality of porphyrin ion-exchange chromatography,11’13 electro synthesis (figure 1). In addition to these phoresis,4’10 and thin-layer chromatogra disorders, there are a number of diseases phy15’16 also have been described. -
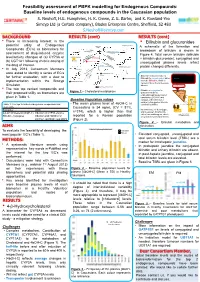
Baseline Levels of Endogenous Compounds in the Caucasian Population S
Feasibility assessment of PBPK modelling for Endogenous Compounds: Baseline levels of endogenous compounds in the Caucasian population S. Neuhoff, H.E. Humphries, H. K. Crewe, Z. E. Barter, and K. Rowland-Yeo Simcyp Ltd (a Certara company), Blades Enterprise Centre, Sheffield, S2 4SU [email protected] BACKGROUND RESULTS (cont) RESULTS (cont) • There is increasing interest in the • Bilirubin and glucuronides 27-Hydroxycholesterol potential utility of Endogenous Cholesterol 1g/day A schematic of the formation and Compounds (EC’s) as biomarkers for CYP7A1 (liver) 500 mg/day CYP27A1 (many tissues) breakdown of bilirubin is shown in assessment of drug-induced enzyme 19 mg/day Figure 4. Total serum bilirubin (bilirubin 7α-Hydroxycholesterol level/activity changes of (a) CYP3A or CYP3A4 + bilirubin glucuronide), conjugated and Bile Acids (b) UGT1A1 following chronic dosing of 4-Hydroxycholesterol unconjugated plasma levels reflect the drug of interest. CYP27A1 (liver and extrahepatic) 24S-Hydroxycholesterol protein changes differently. • In July 2013, Consortium Members Pregnenolone were asked to identify a series of EC’s 4,27-Dihydroxycholesterol Macrophage CYP27A1 • Biliverdin reductase reduces Heme Hemeoxygenase for further evaluation, with a view to 4,24-Dihydroxycholesterol Biliverdin to unconjugated, water- Biliverdin 4,7α-Dihydroxycholesterol insoluble Bilirubin, which is Biliverdin reductase implementation within the Simcyp Bilirubin carried in blood bound to serum Unconjugated bilirubin Simulator. albumin complexed with albumin 3 -

Expanding the Eggshell Colour Gamut: Uroerythrin and Bilirubin from Tinamou (Tinamidae) Eggshells Randy Hamchand1, Daniel Hanley2, Richard O
www.nature.com/scientificreports OPEN Expanding the eggshell colour gamut: uroerythrin and bilirubin from tinamou (Tinamidae) eggshells Randy Hamchand1, Daniel Hanley2, Richard O. Prum3 & Christian Brückner1* To date, only two pigments have been identifed in avian eggshells: rusty-brown protoporphyrin IX and blue-green biliverdin IXα. Most avian eggshell colours can be produced by a mixture of these two tetrapyrrolic pigments. However, tinamou (Tinamidae) eggshells display colours not easily rationalised by combination of these two pigments alone, suggesting the presence of other pigments. Here, through extraction, derivatization, spectroscopy, chromatography, and mass spectrometry, we identify two novel eggshell pigments: yellow–brown tetrapyrrolic bilirubin from the guacamole- green eggshells of Eudromia elegans, and red–orange tripyrrolic uroerythrin from the purplish-brown eggshells of Nothura maculosa. Both pigments are known porphyrin catabolites and are found in the eggshells in conjunction with biliverdin IXα. A colour mixing model using the new pigments and biliverdin reproduces the respective eggshell colours. These discoveries expand our understanding of how eggshell colour diversity is achieved. We suggest that the ability of these pigments to photo- degrade may have an adaptive value for the tinamous. Birds’ eggs are found in an expansive variety of shapes, sizes, and colourings 1. Te diverse array of appearances found across Aves is achieved—in large part—through a combination of structural features, solid or patterned colorations, the use of two diferent dyes, and diferential pigment deposition. Eggshell pigments are embedded within the white calcium carbonate matrix of the egg and within a thin outer proteinaceous layer called the cuticle2–4. Tese pigments are believed to play a key role in crypsis5,6, although other, possibly dynamic 7,8, roles in inter- and intra-species signalling5,9–12 are also possible. -

Phyllobilins – the Abundant Bilin-Type Tetrapyrrolic Catabolites Of
Chemical Society Reviews Phyllobilins – the Abundant Bilin -Type Tetrapyrrolic Catabolites of the Green Plant Pigment Chlorophyll Journal: Chemical Society Reviews Manuscript ID: CS-TRV-02-2014-000079.R1 Article Type: Tutorial Review Date Submitted by the Author: 02-May-2014 Complete List of Authors: Krautler, Bernhard; University of Innsbruck, Institute of Organic Chemistry Page 1 of 30 Chemical Society Reviews Phyllobilins-TutRev-BKräutler 2-May-14 1 Phyllobilins – the Abundant Bilin-type Tetrapyrrolic Catabolites of the Green Plant Pigment Chlorophyll Bernhard Kräutler Institute of Organic Chemistry and Centre of Molecular Biosciences, University of Innsbruck, Innrain 80/82, A-6020 Innsbruck, Austria E-mail: [email protected] Abstract . The seasonal disappearance of the green plant pigment chlorophyll in the leaves of deciduous trees has long been a fascinating biological puzzle. In the course of the last two and a half decades, important aspects of the previously enigmatic breakdown of chlorophyll in higher plants were elucidated. Crucial advances in this field were achieved by the discovery and structure elucidation of tetrapyrrolic chlorophyll catabolites, as well as by complementary biochemical and plant biological studies. Phyllobilins, tetrapyrrolic, bilin-type chlorophyll degradation products, are abundant chlorophyll catabolites, which occur in fall leaves and in ripe fruit. This tutorial review outlines ‘how’ chlorophyll is degraded in higher plants, and gives suggestions as to ‘why’ the plants dispose of their valuable green pigments during senescence and ripening. Insights into chlorophyll breakdown help satisfy basic human curiosity and enlighten school teaching. They contribute to fundamental questions in plant biology and may have practical consequences in agriculture and horticulture. -

ABSTRACT ZHANG, SHAOFEI. Synthesis Of
ABSTRACT ZHANG, SHAOFEI. Synthesis of Bacteriochlorins and the Full Skeleton of Bacteriochlorophylls. (Under the direction of Professor Jonathan S. Lindsey.) Bacteriochlorins, the core macrocycle ring of natural bacteriochlorophylls, are characterized by their ability to absorb near infrared light (700 – 900 nm), which makes them attractive candidates in variety of photophysical studies and applications. The previously established de novo synthesis, which relies on the acid-catalyzed self-condensation of dihydrodipyrrin–acetal, provides access towards diverse bacteriochlorins, but has its limitations. This dissertation describes the development of new strategies for bacteriochlorin synthesis. Firstly, a route towards previously unknown tetra-alkyl bacteriochlorins (e.g., alkyl = Me, or –CH2CO2Me) is established (Ch. 2). Secondly, explorations of synthetic approaches to unsymmetrically substituted bacteriochlorins through electrocyclic reactions of linear tetrapyrrole intermediates are described. Four new unsymmetrically substituted bacteriochlorins and one new tetradehydrocorrin were produced, albeit in low yields (Ch. 3). Thirdly, a new method to construct bacteriochlorin macrocycle with concomitant Nazarov cyclization to form the annulated isocyclic ring, is established. Five new bacteriochlorins, which are closely anlogues of bacteriochlorophyll a, bearing various substituents (alkyl/alkyl, aryl, and alkyl/ester) at positions 2 and 3 and 132 carboalkoxy groups (R = Me or Et) were constructed in 37−61% yield from the bilin analogues -

Phycoerythrin-Specific Bilin Lyase–Isomerase Controls Blue-Green
Phycoerythrin-specific bilin lyase–isomerase controls blue-green chromatic acclimation in marine Synechococcus Animesh Shuklaa, Avijit Biswasb, Nicolas Blotc,d,e, Frédéric Partenskyc,d, Jonathan A. Kartyf, Loubna A. Hammadf, Laurence Garczarekc,d, Andrian Gutua,g, Wendy M. Schluchterb, and David M. Kehoea,h,1 aDepartment of Biology, Indiana University, Bloomington, IN 47405; bDepartment of Biological Sciences, University of New Orleans, New Orleans, LA 70148; cUPMC-Université Paris 06, Station Biologique, 29680 Roscoff, France; dCentre National de la Recherche Scientifique, Unité Mixte de Recherche 7144 Adaptation et Diversité en Milieu Marin, Groupe Plancton Océanique, 29680 Roscoff, France; eClermont Université, Université Blaise Pascal, Unité Mixte de Recherche Centre National de la Recherche Scientifique 6023, Laboratoire Microorganismes: Génome et Environnement, 63000 Clermont-Ferrand, France; fMETACyt Biochemical Analysis Center, Department of Chemistry, Indiana University, Bloomington, IN 47405; gDepartment of Molecular and Cellular Biology, Harvard University, Cambridge, MA 02138; and hIndiana Molecular Biology Institute, Indiana University, Bloomington, IN 47405 Edited by Alexander Namiot Glazer, University of California, Berkeley, CA, and approved October 4, 2012 (received for review July 10, 2012) The marine cyanobacterium Synechococcus is the second most Marine Synechococcus are divided into three major pigment abundant phytoplanktonic organism in the world’s oceans. The types, with type 1 PBS rods containing only PC, type 2 containing ubiquity of this genus is in large part due to its use of a diverse PC and PEI, and type 3 containing PC, PEI, and PEII (4). Type 3 set of photosynthetic light-harvesting pigments called phycobilipro- can be further split into four subtypes (3 A–D)onthebasisofthe teins, which allow it to efficiently exploit a wide range of light ratio of the PUB and PEB chromophores bound to PEs. -

Based Photodynamic Diagnosis of Malignant Brain Tumor: Molecular Design of ABCG2 Inhibitors
Pharmaceutics 2011 , 3, 615-635; doi:10.3390/pharmaceutics3030615 OPEN ACCESS pharmaceutics ISSN 1999-4923 www.mdpi.com/journal/pharmaceutics Review Transporter-Mediated Drug Interaction Strategy for 5-Aminolevulinic Acid (ALA)-Based Photodynamic Diagnosis of Malignant Brain Tumor: Molecular Design of ABCG2 Inhibitors Toshihisa Ishikawa 1,*, Kenkichi Takahashi 2, Naokado Ikeda 2, Yoshinaga Kajimoto 2, Yuichiro Hagiya 3, Shun-ichiro Ogura 3, Shin-ichi Miyatake 2 and Toshihiko Kuroiwa 2 1 Omics Science Center, RIKEN Yokohama Institute, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, 230-0045, Japan 2 Department of Neurosurgery, Osaka Medical College, Osaka 569-8686, Japan 3 Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, Yokohama 226-8501, Japan * Author to whom correspondence should be addressed: E-Mail: [email protected]; Tel.: +81-45-503-9222; Fax: +81-45-503-9216. Received: 19 July 2011; in revised form: 16 August 2011 / Accepted: 9 September 2011 / Published: 14 September 2011 Abstract: Photodynamic diagnosis (PDD) is a practical tool currently used in surgical operation of aggressive brain tumors, such as glioblastoma. PDD is achieved by a photon-induced physicochemical reaction which is induced by excitation of protoporphyrin IX (PpIX) exposed to light. Fluorescence-guided gross-total resection has recently been developed in PDD, where 5-aminolevulinic acid (ALA) or its ester is administered as the precursor of PpIX. ALA induces the accumulation of PpIX, a natural photo-sensitizer, in cancer cells. Recent studies provide evidence that adenosine triphosphate (ATP)-binding cassette (ABC) transporter ABCG2 plays a pivotal role in regulating the cellular accumulation of porphyrins in cancer cells and thereby affects the efficacy of PDD. -

Bilirubin Secretion, Jaundice and Evaluation of Liver Function
Bilirubin Secretion, Jaundice and Evaluation of Liver Function Howard J. Worman, M. D. Evaluation of Liver Disease and Hepatic Function History Physical Examination Laboratory Tests Sometimes Radiological/Nuclear Medicine Sometimes Liver Biopsy 1 Jaundice occurs as a result of excess bilirubin in the blood. It is a hallmark of liver disease but not always present in liver disease. Jaundice occurs when the liver fails to adequately secrete bilirubin from the blood into the bile. To understand how jaundice occurs, you must first understand bilirubin synthesis, metabolism and secretion. 2 Heme Oxygenase Schuller et al. Nature Structural Biology 6, 860 - 867 (1999) Bilivirdin Reductase Kikuchi et al. Nature Structural Biology 8, 221 - 225 (2001) 3 Bilirubin is frequently depicted as a linear tetrapyrrole. However, intramolecular hydrogen bonding fixes it in a rigid structure that blocks exposure of its polar groups to aqueous solvents, making it very insoluble in blood. 4 Bilirubin in Blood is Bound to Albumin: Uptake into Hepatocyte at Basolateral (Sinusoidal) Membrane Some OATP-C? bilirubin stored in cytosol bound to proteins Bilurubin UDP-glucuronosyltransferase is localized to the endo- plasmic reticulum; it catalyzes conjugation to a diglucuronide, making it more water soluble. A: Labeling of periphery of cell hepatocyte nucleus B: Labeling of ER with antibody to UDP-glucuronosyltransferase 5 Alternative RNA splicing of different first exons of UGT1 gives different isoforms with different substrate specificities, some for bilirubin and others