Functional Trade-Off Between Strength and Thermal Capacity of Dermal Armor Insights from Girdled Lizards
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trade in Live Reptiles, Its Impact on Wild Populations, and the Role of the European Market
BIOC-06813; No of Pages 17 Biological Conservation xxx (2016) xxx–xxx Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/bioc Review Trade in live reptiles, its impact on wild populations, and the role of the European market Mark Auliya a,⁎,SandraAltherrb, Daniel Ariano-Sanchez c, Ernst H. Baard d,CarlBrownd,RafeM.Browne, Juan-Carlos Cantu f,GabrieleGentileg, Paul Gildenhuys d, Evert Henningheim h, Jürgen Hintzmann i, Kahoru Kanari j, Milivoje Krvavac k, Marieke Lettink l, Jörg Lippert m, Luca Luiselli n,o, Göran Nilson p, Truong Quang Nguyen q, Vincent Nijman r, James F. Parham s, Stesha A. Pasachnik t,MiguelPedronou, Anna Rauhaus v,DannyRuedaCórdovaw, Maria-Elena Sanchez x,UlrichScheppy, Mona van Schingen z,v, Norbert Schneeweiss aa, Gabriel H. Segniagbeto ab, Ruchira Somaweera ac, Emerson Y. Sy ad,OguzTürkozanae, Sabine Vinke af, Thomas Vinke af,RajuVyasag, Stuart Williamson ah,1,ThomasZieglerai,aj a Department Conservation Biology, Helmholtz Centre for Environmental Conservation (UFZ), Permoserstrasse 15, 04318 Leipzig, Germany b Pro Wildlife, Kidlerstrasse 2, 81371 Munich, Germany c Departamento de Biología, Universidad del Valle de, Guatemala d Western Cape Nature Conservation Board, South Africa e Department of Ecology and Evolutionary Biology,University of Kansas Biodiversity Institute, 1345 Jayhawk Blvd, Lawrence, KS 66045, USA f Bosques de Cerezos 112, C.P. 11700 México D.F., Mexico g Dipartimento di Biologia, Universitá Tor Vergata, Roma, Italy h Amsterdam, The Netherlands -

Description of a New Flat Gecko (Squamata: Gekkonidae: Afroedura) from Mount Gorongosa, Mozambique
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/320043814 Description of a new flat gecko (Squamata: Gekkonidae: Afroedura) from Mount Gorongosa, Mozambique Article in Zootaxa · September 2017 DOI: 10.11646/zootaxa.4324.1.8 CITATIONS READS 2 531 8 authors, including: William R Branch Jennifer Anna Guyton Nelson Mandela University Princeton University 250 PUBLICATIONS 4,231 CITATIONS 7 PUBLICATIONS 164 CITATIONS SEE PROFILE SEE PROFILE Andreas Schmitz Michael Barej Natural History Museum of Geneva Museum für Naturkunde - Leibniz Institute for Research on Evolution and Biodiver… 151 PUBLICATIONS 2,701 CITATIONS 38 PUBLICATIONS 274 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Monitoring and Managing Biodiversity Loss in South-East Africa's Montane Ecosystems View project Ad hoc herpetofauna observations View project All content following this page was uploaded by Jennifer Anna Guyton on 27 September 2017. The user has requested enhancement of the downloaded file. Zootaxa 4324 (1): 142–160 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4324.1.8 http://zoobank.org/urn:lsid:zoobank.org:pub:B4FF9A5F-94A7-4E75-9EC8-B3C382A9128C Description of a new flat gecko (Squamata: Gekkonidae: Afroedura) from Mount Gorongosa, Mozambique WILLIAM R. BRANCH1,2,13, JENNIFER A. GUYTON3, ANDREAS SCHMITZ4, MICHAEL F. BAREJ5, PIOTR NASKRECKI6,7, HARITH FAROOQ8,9,10,11, LUKE VERBURGT12 & MARK-OLIVER RÖDEL5 1Port Elizabeth Museum, P.O. Box 13147, Humewood 6013, South Africa 2Research Associate, Department of Zoology, Nelson Mandela University, P.O. -

SUNGAZERS THREATENED? Sungazers Only Reproduce Every Other Year, and the Sungazer Is Endemic (Only Only Produce One Or Two Offspring
CONSERVATION STATUS: WHY ARE SUNGAZERS THREATENED? Sungazers only reproduce every other year, and The Sungazer is endemic (only only produce one or two offspring. They are viviparous meaning they give birth to live SUNGAZERCordylus giganteus found in one particular country or geographic area) to South Africa. It young. The population is thought to be in OTHER NAMES is found in the highland grasslands decline due to habitat destruction as a result of Giant Zonure, Giant Girdled Lizard or of the north eastern Free State as conversion of grassland to farmland (maize, Ouvolk (Afrikaans - also refers to other well as a small population in south sunflower and other crop farming), illegal Girdled Lizards) western Mpumalanga province. collecting for the pet trade, as well as collection for the muti (traditional medicine) industry. DESCRIPTION The population status is unknown Length but thought to be declining. Conversion/transformation (especially plowing) • up to 35-40cm Globally and nationally the of native grassland is the biggest threat to the Key identification features (adult) Giant Girdled Lizard is classified as species. It has been recorded that animals do not This is the largest of the girdled lizards. It is brown in ECOLOGY Vulnerable (IUCN Red List). Find out seem to return to previously plowed land. colour on the upper surface; merging to straw/yellow more at www.iucnredlist.org colouring along the side of the body and yellow Diet WHAT IS THE EWT DOING TO CONSERVE GIANT underneath. This lizard has four very large, spiny scales on Sungazers eats insects, GIRDLED LIZARDS? the back of the head. -

Notes on the Giant Girdled Lizard Cordylus Giganteus A
British Herpetological Society Bulletin, No. 10, 1984 NOTES ON THE GIANT GIRDLED LIZARD CORDYLUS GIGANTEUS A. SMITH JOHAN MARAIS 23IA Langton Road, Montclair, Durban, South Africa The giant girdled lizard, Cordylus giganteus, is one of South Africa's largest and most impressive Cordylids. Though abundant and well known where it occurs, very little has been written about this lizard. Very popular in zoo and private collections and, until recently, easily obtainable from dealers in Europe and U.S.A., the species is now protected throughout most of its range, and it is therefore unlikely that many more specimens will reach the "pet trade" in future. COMMON NAME Giant girdled lizard; sungazer; Lord Derby's girdled lizard; and, in Afrikaans, sonkyker ("sun- watcher") ouvolk ("old people") or skurwejan. Cordylus giganteus RANGE North-eastern Orange Free State and adjacent southern Transvaal. Possibly occurs in bordering areas of Lesotho in the east and the Cape Province in the south. Branch and Patterson (1975) stated that the presence of Cordylus giganteus in the southern Transvaal "is now doubtful". This species is, in fact, abundant in that region. Cordylus giganteus has recently been found in Natal. SIZE Largest specimens measured by De Waal (1978) during his survey were as follows: a male measuring 204 + 172 = 376mm and a female measuring 205 + 181 = 386mm. 30 HABITAT Flat or sloping, mixed to sour grassveld where it excavates its own burrow. The flattened oblong burrow entrances are well-worn and may face any direction. The burrows average 2-3 metres in length and from 30-45cm in depth. -

Giant Sungazer Lizard, Cordylus Giganteus, in Captivity Gary Fogel Email: [email protected]
Bull. Chicago Herp. Soc. 35(12):277-280, 2000 Observations on the Giant Sungazer Lizard, Cordylus giganteus, in Captivity Gary Fogel Email: [email protected] In the past few years, it has come to my attention that more light, approximately 15 inches from the ground. I furnished a sungazer lizards have been introduced into the commercial pet heating pad in the center of the cage for autumn and winter trade. For this reason I thought it would be a good time to use, and a large dog bowl for water. Hiding places were share my observations and interactions with this species, in the provided in the form of two clay tiles, 24 inches long, cut in hope that others might benefit from my experience in keeping half lengthwise, to serve as burrows. These lizards live in these lizards. As you may be aware, there is still very little open grassland areas, and hide in underground burrows, rough- written on Cordylus giganteus, compared to other, more popu- ly three feet long, either dug by the lizards themselves, or dug lar, types of lizards. The only articles I’ve seen in herpeto- by other animals and adapted for use by the sungazers. The cultural magazines have been Switak (1995) on the genus flooring of my enclosure was floor tile, over which I placed Cordylus as a whole, and Donovan (1997) on sungazers specifi- artificial turf, except for 12 inches at the front of the enclosure. cally. The Vivarium has never published an article on the This is where I placed the water bowl and I hoped was the genus Cordylus, and popularized reptile books are general in place the animals would use for their bathroom area (they tend their information, often contradicting one another on the facts to use the same spot repeatedly for this activity). -

Observations of Infanticide and Cannibalism in Four Species of Cordylid Lizard (Squamata: Cordylidae) in Captivity and the Wild
Herpetology Notes, volume 14: 725-729 (2021) (published online on 21 April 2021) Observations of infanticide and cannibalism in four species of cordylid lizard (Squamata: Cordylidae) in captivity and the wild Daniel van Blerk1,†, Jens Reissig2,†, Julia L. Riley 3,†, John Measey1,*, and James Baxter-Gilbert1 Cannibalism, the consumption of conspecifics, of Africa (Reissig, 2014), from Ethiopia to South Africa is taxonomically widespread and occurs across a (latitudinally) and Angola to Ethiopia (longitudinally). diversity of reptilian species (Polis and Myers, 1985). Here, we present observations of cannibalism by four A long-standing, yet antiquated, perspective views species of cordylid lizard, two from free-living wild cannibalism as an aberrant behaviour (as discussed populations and another two from captive settings. Since in Fox, 1975), but contemporary investigations have the natural history of many cordylid species remains noted its important role in the ecology and evolution of deficient, and several species have been observed to many wild populations (Robbins et al., 2013; Cooper display reasonably high degrees of sociality, like group- et al., 2015; Van Kleek et al., 2018). Examples of this living in Armadillo Lizards, Ouroborus cataphractus include habitat partitioning and optimising resource (Boie, 1828) (Mouton, 2011) and Sungazers, Smaug availability, as seen in juvenile Komodo Dragons, giganteus (Smith, 1844) (Parusnath, 2020), these Varanus komodoensis Ouwens, 1912, taking to the trees observations provide important insights -
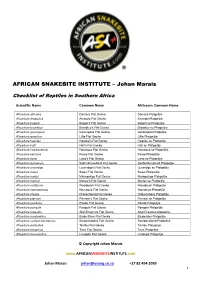
Johan Marais
AFRICAN SNAKEBITE INSTITUTE – Johan Marais Checklist of Reptiles in Southern Africa Scientific Name Common Name Afrikaans Common Name Afroedura africana Damara Flat Gecko Damara Platgeitjie Afroedura amatolica Amatola Flat Gecko Amatola Platgeitjie Afroedura bogerti Bogert's Flat Gecko Bogert se Platgeitjie Afroedura broadleyi Broadley’s Flat Gecko Broadley se Platgeitjie Afroedura gorongosa Gorongosa Flat Gecko Gorongosa Platgeitjie Afroedura granitica Lillie Flat Gecko Lillie Platgeitjie Afroedura haackei Haacke's Flat Gecko Haacke se Platgeitjie Afroedura halli Hall's Flat Gecko Hall se Platgeitjie Afroedura hawequensis Hawequa Flat Gecko Hawequa se Platgeitjie Afroedura karroica Karoo Flat Gecko Karoo Platgeitjie Afroedura langi Lang's Flat Gecko Lang se Platgeitjie Afroedura leoloensis Sekhukhuneland Flat Gecko Sekhukhuneland Platgeitjie Afroedura loveridgei Loveridge's Flat Gecko Loveridge se Platgeitjie Afroedura major Swazi Flat Gecko Swazi Platgeitjie Afroedura maripi Mariepskop Flat Gecko Mariepskop Platgeitjie Afroedura marleyi Marley's Flat Gecko Marley se Platgeitjie Afroedura multiporis Woodbush Flat Gecko Woodbush Platgeijtie Afroedura namaquensis Namaqua Flat Gecko Namakwa Platgeitjie Afroedura nivaria Drakensberg Flat Gecko Drakensberg Platgeitjie Afroedura pienaari Pienaar’s Flat Gecko Pienaar se Platgeitjie Afroedura pondolia Pondo Flat Gecko Pondo Platgeitjie Afroedura pongola Pongola Flat Gecko Pongola Platgeitjie Afroedura rupestris Abel Erasmus Flat Gecko Abel Erasmus platgeitjie Afroedura rondavelica Blyde River -

Patterns of Species Richness, Endemism and Environmental Gradients of African Reptiles
Journal of Biogeography (J. Biogeogr.) (2016) ORIGINAL Patterns of species richness, endemism ARTICLE and environmental gradients of African reptiles Amir Lewin1*, Anat Feldman1, Aaron M. Bauer2, Jonathan Belmaker1, Donald G. Broadley3†, Laurent Chirio4, Yuval Itescu1, Matthew LeBreton5, Erez Maza1, Danny Meirte6, Zoltan T. Nagy7, Maria Novosolov1, Uri Roll8, 1 9 1 1 Oliver Tallowin , Jean-Francßois Trape , Enav Vidan and Shai Meiri 1Department of Zoology, Tel Aviv University, ABSTRACT 6997801 Tel Aviv, Israel, 2Department of Aim To map and assess the richness patterns of reptiles (and included groups: Biology, Villanova University, Villanova PA 3 amphisbaenians, crocodiles, lizards, snakes and turtles) in Africa, quantify the 19085, USA, Natural History Museum of Zimbabwe, PO Box 240, Bulawayo, overlap in species richness of reptiles (and included groups) with the other ter- Zimbabwe, 4Museum National d’Histoire restrial vertebrate classes, investigate the environmental correlates underlying Naturelle, Department Systematique et these patterns, and evaluate the role of range size on richness patterns. Evolution (Reptiles), ISYEB (Institut Location Africa. Systematique, Evolution, Biodiversite, UMR 7205 CNRS/EPHE/MNHN), Paris, France, Methods We assembled a data set of distributions of all African reptile spe- 5Mosaic, (Environment, Health, Data, cies. We tested the spatial congruence of reptile richness with that of amphib- Technology), BP 35322 Yaounde, Cameroon, ians, birds and mammals. We further tested the relative importance of 6Department of African Biology, Royal temperature, precipitation, elevation range and net primary productivity for Museum for Central Africa, 3080 Tervuren, species richness over two spatial scales (ecoregions and 1° grids). We arranged Belgium, 7Royal Belgian Institute of Natural reptile and vertebrate groups into range-size quartiles in order to evaluate the Sciences, OD Taxonomy and Phylogeny, role of range size in producing richness patterns. -

New Verified Nonindigenous Amphibians and Reptiles in Florida Through 2015, with a Summary of Over 152 Years of Introductions
WWW.IRCF.ORG/REPTILESANDAMPHIBIANSJOURNALTABLE OF CONTENTS IRCF REPTILES & IRCF AMPHIBIANS REPTILES • VOL &15, AMPHIBIANS NO 4 • DEC 2008 • 189 23(2):110–143 • AUG 2016 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS INTRODUCED SPECIES FEATURE ARTICLES . Chasing Bullsnakes (Pituophis catenifer sayi) in Wisconsin: New VerifiedOn the Road to Understanding the Nonindigenous Ecology and Conservation of the Midwest’s Giant Serpent ...................... Amphibians Joshua M. Kapfer 190 . The Shared History of Treeboas (Corallus grenadensis) and Humans on Grenada: A Hypothetical Excursion ............................................................................................................................Robert W. Henderson 198 and ReptilesRESEARCH ARTICLES in Florida through 2015, with a . The Texas Horned Lizard in Central and Western Texas ....................... Emily Henry, Jason Brewer, Krista Mougey, and Gad Perry 204 Summary. The Knight Anole of(Anolis equestris over) in Florida 152 Years of Introductions .............................................Brian J. Camposano, Kenneth L. Krysko, Kevin M. Enge, Ellen M. Donlan, and Michael Granatosky 212 1 1 2 3 3 4 Kenneth L. KryskoCONSERVATION, Louis A. Somma ALERT, Dustin C. Smith , Christopher R. Gillette , Daniel Cueva , Joseph A. Wasilewski , 5 6 7 8 9 10 Kevin M. Enge. , Steve A. Johnson , Todd S. Campbell , Jake R. Edwards , Michael R. Rochford , Rhyan Tompkins , World’s Mammals11 in Crisis .............................................................................................................................................................12 -

Causes and Consequences of Body Armour in the Group-Living Lizard, Ouroborus Cataphractus (Cordylidae)
Causes and consequences of body armour in the group-living lizard, Ouroborus cataphractus (Cordylidae) by Chris Broeckhoven Dissertation presented for the degree of Doctor of Philosophy in the Faculty of Science at Stellenbosch University Supervisor: Prof. P. le Fras N. Mouton March 2015 Stellenbosch University https://scholar.sun.ac.za DECLARATION By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. March 2015 Copyright © 2015 Stellenbosch University All rights reserved i Stellenbosch University https://scholar.sun.ac.za ABSTRACT Cordylidae is a family of predominantly rock-dwelling sit-and-wait foraging lizards endemic to southern Africa. The significant variation in spine length and extent of osteoderms among taxa makes the family an excellent model system for studying the evolution of body armour. Specifically, the Armadillo lizard (Ouroborus cataphractus) offers an ideal opportunity to investigate the causes and consequences of body armour. Previous studies have hypothesised that high terrestrial predation pressure, resulting from excursions to termite foraging ports away from the safety of the shelter, has led to the elaboration of body armour and a unique tail-biting behaviour. The reduction in running speed associated with heavy body armour, in turn, appears to have led to the evolution of group-living behaviour to lower the increased aerial predation risk. -

African Herp News
African Herp News Newsletter of the Herpetological Association of Africa Number 55 DECEMBER 2011 HERPETOLOGICAL ASSOCIATION OF AFRICA http://www. wits.ac.za/haa FOUNDED 1965 The HAA is dedicated to the study and conservation of African reptiles and amphibians. Membership is open to anyone with an interest in the African herpetofauna. Members receive the Association’s journal, African Journal of Herpetology (which publishes review papers, research articles, and short communications – subject to peer review) and African Herp News , the Newsletter (which includes short communications, natural history notes, geographical distribution notes, herpetological survey reports, venom and snakebite notes, book reviews, bibliographies, husbandry hints, announcements and news items). NEWSLETTER EDITOR ’S NOTE Articles shall be considered for publication provided that they are original and have not been published elsewhere. Articles will be submitted for peer review at the Editor’s discretion. Authors are requested to submit manuscripts by e-mail in MS Word ‘.doc’ or ‘.docx’ format. COPYRIGHT: Articles published in the Newsletter are copyright of the Herpetological Association of Africa and may not be reproduced without permission of the Editor. The views and opinions expressed in articles are not necessarily those of the Editor . COMMITTEE OF THE HERPETOLOGICAL ASSOCIATION OF AFRICA CHAIRMAN Aaron Bauer, Department of Biology, Villanova University, 800 Lancaster Avenue, Villanova, Pennsylvania 19085, USA. [email protected] SECRETARY Jeanne Tarrant, African Amphibian Conservation Research Group, NWU. 40A Hilltop Road, Hillcrest 3610, South Africa. [email protected] TREASURER Abeda Dawood, National Zoological Gardens, Corner of Boom and Paul Kruger Streets, Pretoria 0002, South Africa. [email protected] JOURNAL EDITOR John Measey, Applied Biodiversity Research, Kirstenbosch Research Centre, South African Biodiversity Institute, P/Bag X7, Claremont 7735, South Africa. -

A Taxonomic Revision of the South-Eastern Dragon Lizards of the Smaug Warreni (Boulenger) Species Complex in Southern Africa, Wi
A taxonomic revision of the south-eastern dragon lizards of the Smaug warreni (Boulenger) species complex in southern Africa, with the description of a new species (Squamata: Cordylidae) Michael F. Bates1,2,* and Edward L. Stanley3,* 1 Department of Herpetology, National Museum, Bloemfontein, South Africa 2 Department of Zoology and Entomology, University of the Free State, Bloemfontein, South Africa 3 Department of Herpetology, Florida Museum of Natural History, Gainesville, FL, USA * These authors contributed equally to this work. ABSTRACT A recent multilocus molecular phylogenyofthelargedragonlizardsofthegenus Smaug Stanley et al. (2011) recovered a south-eastern clade of two relatively lightly- armoured, geographically-proximate species (Smaug warreni (Boulenger, 1908) and S. barbertonensis (Van Dam, 1921)). Unexpectedly, S. barbertonensis was found to be paraphyletic, with individuals sampled from northern Eswatini (formerly Swaziland) being more closely related to S. warreni than to S. barbertonensis from the type locality of Barberton in Mpumalanga Province, South Africa. Examination of voucher specimens used for the molecular analysis, as well as most other available museum material of the three lineages, indicated that the ‘Eswatini’ lineage—including populations in a small area on the northern Eswatini–Mpumalanga border, and northern KwaZulu–Natal Province in South Africa—was readily distinguishable from S. barbertonensis sensu stricto (and S. warreni) by its unique dorsal, lateral and ventral colour patterns. In order to further assess the taxonomic status of the three populations, a detailed Submitted 26 September 2019 Accepted 7 January 2020 morphological analysis was conducted. Multivariate analyses of scale counts and Published 25 March 2020 body dimensions indicated that the ‘Eswatini’ lineage and S.