A Review of Cordylus Machadoi (Squamata: Cordylidae) in Southwestern Angola, with the Description of a New Species from the Pro-Namib Desert
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nyika and Vwaza Reptiles & Amphibians Checklist
LIST OF REPTILES AND AMPHIBIANS OF NYIKA NATIONAL PARK AND VWAZA MARSH WILDLIFE RESERVE This checklist of all reptile and amphibian species recorded from the Nyika National Park and immediate surrounds (both in Malawi and Zambia) and from the Vwaza Marsh Wildlife Reserve was compiled by Dr Donald Broadley of the Natural History Museum of Zimbabwe in Bulawayo, Zimbabwe, in November 2013. It is arranged in zoological order by scientific name; common names are given in brackets. The notes indicate where are the records are from. Endemic species (that is species only known from this area) are indicated by an E before the scientific name. Further details of names and the sources of the records are available on request from the Nyika Vwaza Trust Secretariat. REPTILES TORTOISES & TERRAPINS Family Pelomedusidae Pelusios rhodesianus (Variable Hinged Terrapin) Vwaza LIZARDS Family Agamidae Acanthocercus branchi (Branch's Tree Agama) Nyika Agama kirkii kirkii (Kirk's Rock Agama) Vwaza Agama armata (Eastern Spiny Agama) Nyika Family Chamaeleonidae Rhampholeon nchisiensis (Nchisi Pygmy Chameleon) Nyika Chamaeleo dilepis (Common Flap-necked Chameleon) Nyika(Nchenachena), Vwaza Trioceros goetzei nyikae (Nyika Whistling Chameleon) Nyika(Nchenachena) Trioceros incornutus (Ukinga Hornless Chameleon) Nyika Family Gekkonidae Lygodactylus angularis (Angle-throated Dwarf Gecko) Nyika Lygodactylus capensis (Cape Dwarf Gecko) Nyika(Nchenachena), Vwaza Hemidactylus mabouia (Tropical House Gecko) Nyika Family Scincidae Trachylepis varia (Variable Skink) Nyika, -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

Description of a New Flat Gecko (Squamata: Gekkonidae: Afroedura) from Mount Gorongosa, Mozambique
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/320043814 Description of a new flat gecko (Squamata: Gekkonidae: Afroedura) from Mount Gorongosa, Mozambique Article in Zootaxa · September 2017 DOI: 10.11646/zootaxa.4324.1.8 CITATIONS READS 2 531 8 authors, including: William R Branch Jennifer Anna Guyton Nelson Mandela University Princeton University 250 PUBLICATIONS 4,231 CITATIONS 7 PUBLICATIONS 164 CITATIONS SEE PROFILE SEE PROFILE Andreas Schmitz Michael Barej Natural History Museum of Geneva Museum für Naturkunde - Leibniz Institute for Research on Evolution and Biodiver… 151 PUBLICATIONS 2,701 CITATIONS 38 PUBLICATIONS 274 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Monitoring and Managing Biodiversity Loss in South-East Africa's Montane Ecosystems View project Ad hoc herpetofauna observations View project All content following this page was uploaded by Jennifer Anna Guyton on 27 September 2017. The user has requested enhancement of the downloaded file. Zootaxa 4324 (1): 142–160 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4324.1.8 http://zoobank.org/urn:lsid:zoobank.org:pub:B4FF9A5F-94A7-4E75-9EC8-B3C382A9128C Description of a new flat gecko (Squamata: Gekkonidae: Afroedura) from Mount Gorongosa, Mozambique WILLIAM R. BRANCH1,2,13, JENNIFER A. GUYTON3, ANDREAS SCHMITZ4, MICHAEL F. BAREJ5, PIOTR NASKRECKI6,7, HARITH FAROOQ8,9,10,11, LUKE VERBURGT12 & MARK-OLIVER RÖDEL5 1Port Elizabeth Museum, P.O. Box 13147, Humewood 6013, South Africa 2Research Associate, Department of Zoology, Nelson Mandela University, P.O. -

Notes on the Giant Girdled Lizard Cordylus Giganteus A
British Herpetological Society Bulletin, No. 10, 1984 NOTES ON THE GIANT GIRDLED LIZARD CORDYLUS GIGANTEUS A. SMITH JOHAN MARAIS 23IA Langton Road, Montclair, Durban, South Africa The giant girdled lizard, Cordylus giganteus, is one of South Africa's largest and most impressive Cordylids. Though abundant and well known where it occurs, very little has been written about this lizard. Very popular in zoo and private collections and, until recently, easily obtainable from dealers in Europe and U.S.A., the species is now protected throughout most of its range, and it is therefore unlikely that many more specimens will reach the "pet trade" in future. COMMON NAME Giant girdled lizard; sungazer; Lord Derby's girdled lizard; and, in Afrikaans, sonkyker ("sun- watcher") ouvolk ("old people") or skurwejan. Cordylus giganteus RANGE North-eastern Orange Free State and adjacent southern Transvaal. Possibly occurs in bordering areas of Lesotho in the east and the Cape Province in the south. Branch and Patterson (1975) stated that the presence of Cordylus giganteus in the southern Transvaal "is now doubtful". This species is, in fact, abundant in that region. Cordylus giganteus has recently been found in Natal. SIZE Largest specimens measured by De Waal (1978) during his survey were as follows: a male measuring 204 + 172 = 376mm and a female measuring 205 + 181 = 386mm. 30 HABITAT Flat or sloping, mixed to sour grassveld where it excavates its own burrow. The flattened oblong burrow entrances are well-worn and may face any direction. The burrows average 2-3 metres in length and from 30-45cm in depth. -

Muscle Biochemistry and Body Shape Explain Ontogenetic Variation of Anti
© 2016. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2016) 219, 1649-1658 doi:10.1242/jeb.130740 RESEARCH ARTICLE Beyond body size: muscle biochemistry and body shape explain ontogenetic variation of anti-predatory behaviour in the lizard Salvator merianae Fábio Cury de Barros1, JoséEduardo de Carvalho2, Augusto Shinya Abe3 and Tiana Kohlsdorf1,* ABSTRACT When facing a predator and after choosing among possible anti- Anti-predatory behaviour evolves under the strong action of natural predatory strategies, animals are likely to adjust the characteristics selection because the success of individuals avoiding predation and intensity of the elected behavioural response according to the essentially defines their fitness. Choice of anti-predatory strategies is perceived level of predation risk (Brown et al., 2006; Greene, 1988; defined by prey characteristics as well as environmental temperature. Martín and López, 2003; Ydenberg and Dill, 1986). Many factors An additional dimension often relegated in this multilevel equation is might influence the choice and intensity of the elected anti- the ontogenetic component. In the tegu Salvator merianae, adults run predatory tactic, such as local conditions [i.e. environmental away from predators at high temperatures but prefer fighting when it is temperature, amount of light/period of day and vegetation cover/ cold, whereas juveniles exhibit the same flight strategy within a wide terrain characteristics (Savino and Stein, 1989; Christensen and thermal range. Here, we integrate physiology and morphology to Persson, 1993; Brodie and Russell, 1999; Shine et al., 2000, 2003; understand ontogenetic variation in the temperature-dependent shift Schulte et al., 2004; Durso and Mullin, 2014)] or type and density of of anti-predatory behaviour in these lizards. -

Giant Sungazer Lizard, Cordylus Giganteus, in Captivity Gary Fogel Email: [email protected]
Bull. Chicago Herp. Soc. 35(12):277-280, 2000 Observations on the Giant Sungazer Lizard, Cordylus giganteus, in Captivity Gary Fogel Email: [email protected] In the past few years, it has come to my attention that more light, approximately 15 inches from the ground. I furnished a sungazer lizards have been introduced into the commercial pet heating pad in the center of the cage for autumn and winter trade. For this reason I thought it would be a good time to use, and a large dog bowl for water. Hiding places were share my observations and interactions with this species, in the provided in the form of two clay tiles, 24 inches long, cut in hope that others might benefit from my experience in keeping half lengthwise, to serve as burrows. These lizards live in these lizards. As you may be aware, there is still very little open grassland areas, and hide in underground burrows, rough- written on Cordylus giganteus, compared to other, more popu- ly three feet long, either dug by the lizards themselves, or dug lar, types of lizards. The only articles I’ve seen in herpeto- by other animals and adapted for use by the sungazers. The cultural magazines have been Switak (1995) on the genus flooring of my enclosure was floor tile, over which I placed Cordylus as a whole, and Donovan (1997) on sungazers specifi- artificial turf, except for 12 inches at the front of the enclosure. cally. The Vivarium has never published an article on the This is where I placed the water bowl and I hoped was the genus Cordylus, and popularized reptile books are general in place the animals would use for their bathroom area (they tend their information, often contradicting one another on the facts to use the same spot repeatedly for this activity). -

The Female Reproductive Cycle of the Lizard, Cordylus Polyzonus Polyzonus (Sauria: Cordylidae) in the Orange Free State
S. Afr. J. Zoo!. 1989,24(4) 263 The female reproductive cycle of the lizard, Cordylus polyzonus polyzonus (Sauria: Cordylidae) in the Orange Free State J.H. van Wyk* National Museum, P.O. Box 266, Bloemfontein, 9300 Republic of South Africa Received 13 November 1987; accepted 24 February 1989 Winter reproductive activity is exhibited by females of the vIviparous lizard, Cordy/us p.po/yzonus. Vitellogenesis commences in May (autumn), continuing throughout the winter months with ovulation in October. Females are pregnant during summer and give birth in late summer (February). Clutch size is positively correlated to female body size. During embryonic development, ovarian follicles remain small and translucent and the corpora lutea progressively decrease in size until parturition occurs. Embryonic growth was characterized by a concomitant decline in embryonic yolk mass. Although considerable variation was observed in the fat body mass, maximal fat body mass peaked in winter (June) and in mid-summer (December). The onset of vitellogenesis corresponded to declining photoperiod and environmental temperatures. Die lewendbarende akkedis, Cordy/us p. po/yzonus toon voortplantingsaktiwiteit gedurende die wintermaande. Dooierneerlegging begin gedurende Mei (herts) en duur voort deur die wintermaande tot ovulasie in Oktober. Wyfies is verwagtend gedurende die somer en kleintjies word in die laat-somer (Februarie) gebore. Eiergetal is positief gekorreleerd met die liggaamsgrootte van die wyfie. Gedurende embrionale ontwikkeling bly die ovariumfollikels klein en deursigtig en die corpora lutea verklein voor geboorte in Februarie. Embrionale groei gaan gepaard met 'n afname in die embrionale dooiermassa. Alhoewel aansienlike variasie in die massa van die vetliggame voorkom, is maksimum vetliggaam-massa gedurende winter- (Junie) en somermaande (Desember) waargeneem. -

Observations of Infanticide and Cannibalism in Four Species of Cordylid Lizard (Squamata: Cordylidae) in Captivity and the Wild
Herpetology Notes, volume 14: 725-729 (2021) (published online on 21 April 2021) Observations of infanticide and cannibalism in four species of cordylid lizard (Squamata: Cordylidae) in captivity and the wild Daniel van Blerk1,†, Jens Reissig2,†, Julia L. Riley 3,†, John Measey1,*, and James Baxter-Gilbert1 Cannibalism, the consumption of conspecifics, of Africa (Reissig, 2014), from Ethiopia to South Africa is taxonomically widespread and occurs across a (latitudinally) and Angola to Ethiopia (longitudinally). diversity of reptilian species (Polis and Myers, 1985). Here, we present observations of cannibalism by four A long-standing, yet antiquated, perspective views species of cordylid lizard, two from free-living wild cannibalism as an aberrant behaviour (as discussed populations and another two from captive settings. Since in Fox, 1975), but contemporary investigations have the natural history of many cordylid species remains noted its important role in the ecology and evolution of deficient, and several species have been observed to many wild populations (Robbins et al., 2013; Cooper display reasonably high degrees of sociality, like group- et al., 2015; Van Kleek et al., 2018). Examples of this living in Armadillo Lizards, Ouroborus cataphractus include habitat partitioning and optimising resource (Boie, 1828) (Mouton, 2011) and Sungazers, Smaug availability, as seen in juvenile Komodo Dragons, giganteus (Smith, 1844) (Parusnath, 2020), these Varanus komodoensis Ouwens, 1912, taking to the trees observations provide important insights -
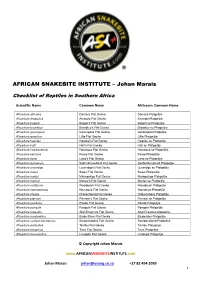
Johan Marais
AFRICAN SNAKEBITE INSTITUTE – Johan Marais Checklist of Reptiles in Southern Africa Scientific Name Common Name Afrikaans Common Name Afroedura africana Damara Flat Gecko Damara Platgeitjie Afroedura amatolica Amatola Flat Gecko Amatola Platgeitjie Afroedura bogerti Bogert's Flat Gecko Bogert se Platgeitjie Afroedura broadleyi Broadley’s Flat Gecko Broadley se Platgeitjie Afroedura gorongosa Gorongosa Flat Gecko Gorongosa Platgeitjie Afroedura granitica Lillie Flat Gecko Lillie Platgeitjie Afroedura haackei Haacke's Flat Gecko Haacke se Platgeitjie Afroedura halli Hall's Flat Gecko Hall se Platgeitjie Afroedura hawequensis Hawequa Flat Gecko Hawequa se Platgeitjie Afroedura karroica Karoo Flat Gecko Karoo Platgeitjie Afroedura langi Lang's Flat Gecko Lang se Platgeitjie Afroedura leoloensis Sekhukhuneland Flat Gecko Sekhukhuneland Platgeitjie Afroedura loveridgei Loveridge's Flat Gecko Loveridge se Platgeitjie Afroedura major Swazi Flat Gecko Swazi Platgeitjie Afroedura maripi Mariepskop Flat Gecko Mariepskop Platgeitjie Afroedura marleyi Marley's Flat Gecko Marley se Platgeitjie Afroedura multiporis Woodbush Flat Gecko Woodbush Platgeijtie Afroedura namaquensis Namaqua Flat Gecko Namakwa Platgeitjie Afroedura nivaria Drakensberg Flat Gecko Drakensberg Platgeitjie Afroedura pienaari Pienaar’s Flat Gecko Pienaar se Platgeitjie Afroedura pondolia Pondo Flat Gecko Pondo Platgeitjie Afroedura pongola Pongola Flat Gecko Pongola Platgeitjie Afroedura rupestris Abel Erasmus Flat Gecko Abel Erasmus platgeitjie Afroedura rondavelica Blyde River -

Patterns of Species Richness, Endemism and Environmental Gradients of African Reptiles
Journal of Biogeography (J. Biogeogr.) (2016) ORIGINAL Patterns of species richness, endemism ARTICLE and environmental gradients of African reptiles Amir Lewin1*, Anat Feldman1, Aaron M. Bauer2, Jonathan Belmaker1, Donald G. Broadley3†, Laurent Chirio4, Yuval Itescu1, Matthew LeBreton5, Erez Maza1, Danny Meirte6, Zoltan T. Nagy7, Maria Novosolov1, Uri Roll8, 1 9 1 1 Oliver Tallowin , Jean-Francßois Trape , Enav Vidan and Shai Meiri 1Department of Zoology, Tel Aviv University, ABSTRACT 6997801 Tel Aviv, Israel, 2Department of Aim To map and assess the richness patterns of reptiles (and included groups: Biology, Villanova University, Villanova PA 3 amphisbaenians, crocodiles, lizards, snakes and turtles) in Africa, quantify the 19085, USA, Natural History Museum of Zimbabwe, PO Box 240, Bulawayo, overlap in species richness of reptiles (and included groups) with the other ter- Zimbabwe, 4Museum National d’Histoire restrial vertebrate classes, investigate the environmental correlates underlying Naturelle, Department Systematique et these patterns, and evaluate the role of range size on richness patterns. Evolution (Reptiles), ISYEB (Institut Location Africa. Systematique, Evolution, Biodiversite, UMR 7205 CNRS/EPHE/MNHN), Paris, France, Methods We assembled a data set of distributions of all African reptile spe- 5Mosaic, (Environment, Health, Data, cies. We tested the spatial congruence of reptile richness with that of amphib- Technology), BP 35322 Yaounde, Cameroon, ians, birds and mammals. We further tested the relative importance of 6Department of African Biology, Royal temperature, precipitation, elevation range and net primary productivity for Museum for Central Africa, 3080 Tervuren, species richness over two spatial scales (ecoregions and 1° grids). We arranged Belgium, 7Royal Belgian Institute of Natural reptile and vertebrate groups into range-size quartiles in order to evaluate the Sciences, OD Taxonomy and Phylogeny, role of range size in producing richness patterns. -

New Verified Nonindigenous Amphibians and Reptiles in Florida Through 2015, with a Summary of Over 152 Years of Introductions
WWW.IRCF.ORG/REPTILESANDAMPHIBIANSJOURNALTABLE OF CONTENTS IRCF REPTILES & IRCF AMPHIBIANS REPTILES • VOL &15, AMPHIBIANS NO 4 • DEC 2008 • 189 23(2):110–143 • AUG 2016 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS INTRODUCED SPECIES FEATURE ARTICLES . Chasing Bullsnakes (Pituophis catenifer sayi) in Wisconsin: New VerifiedOn the Road to Understanding the Nonindigenous Ecology and Conservation of the Midwest’s Giant Serpent ...................... Amphibians Joshua M. Kapfer 190 . The Shared History of Treeboas (Corallus grenadensis) and Humans on Grenada: A Hypothetical Excursion ............................................................................................................................Robert W. Henderson 198 and ReptilesRESEARCH ARTICLES in Florida through 2015, with a . The Texas Horned Lizard in Central and Western Texas ....................... Emily Henry, Jason Brewer, Krista Mougey, and Gad Perry 204 Summary. The Knight Anole of(Anolis equestris over) in Florida 152 Years of Introductions .............................................Brian J. Camposano, Kenneth L. Krysko, Kevin M. Enge, Ellen M. Donlan, and Michael Granatosky 212 1 1 2 3 3 4 Kenneth L. KryskoCONSERVATION, Louis A. Somma ALERT, Dustin C. Smith , Christopher R. Gillette , Daniel Cueva , Joseph A. Wasilewski , 5 6 7 8 9 10 Kevin M. Enge. , Steve A. Johnson , Todd S. Campbell , Jake R. Edwards , Michael R. Rochford , Rhyan Tompkins , World’s Mammals11 in Crisis .............................................................................................................................................................12 -

An Overview of Pet Reptile Species and Proper Hand
Exotics — Reptiles and Amphibians ______________________________________________________________________________________________ AN OVERVIEW OF PET REPTILE SPECIES Many reptiles are still caught from the wild, and AND PROPER HANDLING shipped to distributors who then supply pet stores. For one animal that makes it through that journey, many if Jean A. Paré, DMV, DVSc, Diplomate ACZM not most, will die. Sometimes whole shipments are lost. College of Veterinary Medicine Wild-caught animals are often fractious and do not adapt Texas A&M University, College Station, TX well to captivity. They come with a parasite burden that may well become overbearing with the stress and the Reptiles are a successful group of ectothermic, scaled confinement of captivity. Not only does buying a captive- vertebrates that are present on all continents except bred reptile help discourage the wild-caught trade, but Antarctica. Taxonomic debates are ongoing and new captive-bred animals are better-adapted, accept reptile species are discovered every year, but it is a handling much better, are less finicky eaters, have fewer reasonable estimate that there are over 7,500 extant parasites, and live longer and healthier lives as a rule species of reptiles, among which roughly 4,500 are than wild-caught specimens. It is incumbent upon us to lizards, 3,000 are snakes, 300 are turtles, 23 are advise potential reptile owners to seek captive-bred crocodilians, and 2 species of tuataras. The world being animals. Some animals are sold under misleading what it is, with the dwindling of habitats and the appellations, such as “farm-raised” or “captive-raised” increasing encroachment of humans on the remaining reptiles, which may only mean that they were maintained wilderness, there are numerous species of reptiles that captive (for weeks to months) after being caught in the are threatened or endangered, often critically.