Chapter 20: Peripheral Arterial Disease, Foot Ulcers, Lower Extremity Amputations, and Diabetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Documentationand Coding Tips: Peripheral Vascular Disease
Documentation and Coding: Peripheral Vascular Disease (PVD)/ Peripheral Arterial Disease (PAD) Created May 2020 At Healthfirst, we are committed to helping providers accurately document and code their patients’ health records. Proper ICD-10 coding can provide a comprehensive view of a patient’s overall health. This tip sheet offers guidance on how to submit a diagnosis code with greater specificity for Peripheral Vascular Disease (PVD)/Peripheral Arterial Disease (PAD). The risk factors for peripheral vascular disease are similar to those for coronary artery disease. The terms arteriosclerosis and atherosclerosis may be used interchangeably for coding and documentation purposes. When completing documentation and coding, you should keep in mind the following: Type of graft Laterality (left, right, or bilateral) and side(s) affected by the complicating condition Type of bypass Any educational information provided to the patient Location of vein or artery graft affected Treatment plan, orders, testing, prescriptions, and referrals Complications like claudication, ulceration, or chest pain Updated status of condition (stable, improved, and/or worsening) Graft, Bypass, Location, Complications, and Laterality Use the chart below to ensure that you are coding properly. Note that this is not an inclusive list of codes and that you are required to have six digits for your diagnosis code. The location and laterality will help determine the fifth and sixth digits for the codes. Type of Graft Location/Laterality Description of Graft ICD-10-CM Without -

Footprints an Informational Newsletter for Patients of APMA Member Podiatrists October 2017
footprints an informational newsletter for patients of APMA member podiatrists october 2017 special EDITION include a DPM for diabetes prevention & Manage Ment According to the CDC, more than 100 million US adults are living with either diabetes or prediabetes. If you have diabetes or prediabetes, it is essential to include a podiatrist for proper diabetes prevention and management. In fact, podiatrists can reduce amputation rates up to 80 percent. In order for you not to become a part of that statistic, here are a few simple things you can do to keep your risk for diabetic ulcers and amputation low: Inspect feet daily. Check your Don’t go barefoot. Don’t go 1 feet and toes every day for 5 without shoes, even in your cuts, bruises, sores, or changes to own home. The risk of cuts and the toenails, such as thickening or infection is too great for those discoloration. with diabetes. Wear thick, soft socks. Avoid Never try to remove calluses, 2 socks with seams, which could 6 corns, or warts by yourself. rub and cause blisters or other Over-the-counter products can burn skin injuries. the skin and cause irreparable damage to the foot for people Exercise. Walking can keep with diabetes. 3 weight down and improve circulation. Be sure to wear See a podiatrist. Regular appropriate athletic shoes when 7 checkups by a podiatrist— exercising. at least annually—are the best WALKING CAN KEEP way to ensure that your feet WEIGHT DOWN AND Have new shoes properly remain healthy. 4 measured and fitted. Foot size IMPROVE CIRCULATION. -
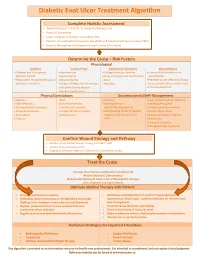
Diabetic Foot Ulcer Treatment Algorithm
Diabetic Foot Ulcer Treatment Algorithm Complete Holistic Assessment Medical/Surgical History & Co-morbidity Management Physical Examination Lower Leg (LLA) including monofilament test Perform Arterial Brachial Pressure Index (ABPI) and Toe Brachial Pressure Index (TBPI) Diabetic Management (Glycemic and Lipid Control & Nutrition) Determine the Cause – Risk Factors Physiological Diabetes Vascular Flow Autoimmune Disorders Wound History Diabetes and sub-optimal Hypertension Collagen vascular diseases History of foot infections or glycemic control Heart disease Immunosuppressant medications osteomyelitis Neuropathic changes with lack of Hyperlipidemia Gout Presence of toe infections (fungal protective sensation History of deep vein thrombosis Vasculitis or bacterial), callous and/or corns Peripheral artery disease Previous ulceration Venous Insufficiency Physical Limitations Socioeconomic/Self-Management Obesity Gait Smoking Lack of awareness for self-care Foot deformity Nutritional deficits Inadequate foot Inadequate hygiene Decreased level of activity Limited joint mobility wear/offloading devices Unsafe home environment Visual disturbances Congenital abnormalities Lack/Inability to afford diabetic Alcohol/drug abuse Amputation Osteoporosis supplies, foot care and foot Decreased Cognitive Ability Trauma wear Depression Financial insecurity Decreased level of activity Confirm Wound Etiology and Pathway Results of LLA, Monofilament Testing and ABPI/ TBPI Results of wound assessment Diagnostic -

Associations Between Nutrients and Foot Ulceration in Diabetes: a Systematic Review
nutrients Review Associations between Nutrients and Foot Ulceration in Diabetes: A Systematic Review Nada Bechara 1,2,3, Jenny E. Gunton 2,3,4,* , Victoria Flood 5,6 , Tien-Ming Hng 1 and Clare McGloin 1 1 Department of Diabetes and Endocrinology, Blacktown-Mt Druitt Hospital, Blacktown, NSW 2148, Australia; [email protected] (N.B.); [email protected] (T.-M.H.); [email protected] (C.M.) 2 Westmead Hospital, Sydney Medical School, Faculty of Medicine and Health, The University of Sydney, Westmead, NSW 2145, Australia 3 Centre for Diabetes, Obesity and Endocrinology Research (CDOER), The Westmead Institute for Medical Research, The University of Sydney, Westmead, NSW 2145, Australia 4 Garvan Institute of Medical Research, Darlinghurst, NSW 2010, Australia 5 Westmead Hospital, Research and Education Network, Western Sydney Local Health District, Westmead, NSW 2145, Australia; [email protected] 6 Faculty of Medicine and Health, Sydney School of Health Sciences, The University of Sydney, Camperdown, NSW 2006, Australia * Correspondence: [email protected] Abstract: We reviewed the literature to evaluate potential associations between vitamins, nutrients, nutritional status or nutritional interventions and presence or healing of foot ulceration in diabetes. Embase, Medline, PubMed, and the Cochrane Library were searched for studies published prior to September 2020. We assessed eligible studies for the association between nutritional status or interventions and foot ulcers. Fifteen studies met the inclusion criteria and were included in this Citation: Bechara, N.; Gunton, J.E.; review. Overall, there is a correlation between poor nutritional status and the presence of foot Flood, V.; Hng, T.-M.; McGloin, C. -

Report on the Cooperative Study of Intracranial Aneurysms And
Report on the Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage SECTION VIII, Part I Results of the Treatment of Intracranial Aneurysms by Occlusion of the Carotid Artery in the Neck HIRO NISHIOKA, M.D.* Introduction In a late postoperative study of retinal artery CCLUSION of the cervical portion of the pressures in 13 patients with carotid liga- carotid artery has been employed tion, Christiansson '6~ found eight patients O since 1885 as a definitive treatment who had maintained pressure drops of 20 per for intracranial aneurysm. The resultant re- cent or more over a period of from 1 to 13 duction of intra-arterial pressure is expected years. The observation that there was stasis to reduce the likelihood of subsequent of blood within the aneuwsmal sac after hemorrhage. The alteration of blood flow carotid occlusion was made at angiography characteristics within the aneurysmal sac by Eeker and Riemenschneider '51. During may encourage thrombosis with organization digital carotid occlusion, they found that and fibrosis, which would strengthen the wall Diodrast remained within the sac for over a or obliterate the sac. That pressure can be minute, and, in the same patient, angiog- reduced effectively in the internal carotid raphy performed one week after partial artery by proximal internal or common ca- occlusion of the common carotid by a tan- rotid occlusion has been substantiated amply talum clip showed no filling of the aneurysm. by the works of many authors. However, Aneurysms may decrease visibly in size or pressure reductions distal to the bifurcation become progressively thrombosed after ca- of the internal carotid artery following ca- rotid ligation. -

Vasculitis: Pearls for Early Diagnosis and Treatment of Giant Cell Arteritis
Vasculitis: Pearls for early diagnosis and treatment of Giant Cell Arteritis Mary Beth Humphrey, MD, PhD Professor of Medicine McEldowney Chair of Immunology [email protected] Office Phone: 405 271-8001 ext 35290 October 2019 Relevant Disclosure and Resolution Under Accreditation Council for Continuing Medical Education guidelines disclosure must be made regarding relevant financial relationships with commercial interests within the last 12 months. Mary Beth Humphrey I have no relevant financial relationships or affiliations with commercial interests to disclose. Experimental or Off-Label Drug/Therapy/Device Disclosure I will be discussing experimental or off-label drugs, therapies and/or devices that have not been approved by the FDA. Objectives • To recognize early signs of vasculitis. • To discuss Tocilizumab (IL-6 inhibitor) as a new treatment option for temporal arteritis. • To recognize complications of vasculitis and therapies. Professional Practice Gap Gap 1: Application of imaging recommendations in large vessel vasculitis Gap 2: Application of tocilizimab in treatment of giant cell vasculitis Cranial Symptoms Aortic Vision loss Aneurysm GCA Arm PMR Claudication FUO Which is not a risk factor or temporal arteritis? A. Smoking B. Female sex C. Diabetes D. Northern European ancestry E. Age Which is not a risk factor or temporal arteritis? A. Smoking B. Female sex C. Diabetes D. Northern European ancestry E. Age Giant Cell Arteritis • Most common form of systemic vasculitis in adults – Incidence: ~ 1/5,000 persons > 50 yrs/year – Lifetime risk: 1.0% (F) 0.5% (M) • Cause: unknown At risk: Women (80%) > men (20%) Northern European ancestry>>>AA>Hispanics Age: average age at onset ~73 years Smoking: 6x increased risk Kermani TA, et al Ann Rheum Dis. -

Jordan M. Garrison, Md Facs Fasmbs What Are We Talking About?
PERIPHERAL VASCULAR DISEASE JORDAN M. GARRISON, MD FACS FASMBS WHAT ARE WE TALKING ABOUT? Peripheral Arterial Disease (PAD) • Near or Complete obstruction of > 1 Peripheral Artery Peripheral Venous Reflux Disease • Varicose Veins • Chronic Venous Stasis Ulcer Disease • Phlegmasia Cerulia Dolans or Alba Dolans (Milk Leg) • Deep Vein Thrombosis and Pulmonary Embolus Disease Coronary Artery Disease ◦ Myocardial Infarct Aneurysmal Disease • Aortic • Popliteal Cerebral and Carotid Artery Disease • Stroke • TIA Renal Vascular Hypertensive Disease • High Blood Pressure • Kidney Failure Peripheral Arterial Disease PREVELANCE Males > Females 10% of People over 60 Race • AA double the rate of all other ethnicities High Income Countries Peripheral Arterial Disease RISK FACTORS Smoking is the #1 risk factor Diabetes Mellitus Diet Obesity ETOH Cholesterol Heredity Lifestyle Homocysteine, C Reactive Protein, Fibrinogen Arterial Anatomy Lower Extremity Anatomy Plaque Formation Plaque Peripheral Arterial Disease Claudication • Disabling • Intestinal Limb Threatening Ischemia(LTI) Critical Limb Ischemia(CLI) • Rest Pain • Tissue Loss • Gangrene Disabling Claudication Intestinal Ischemia Intestinal Claudication Peripheral Arterial Disease Claudication • Disabling • Intestinal Limb Threatening Ischemia(LTI) Critical Limb Ischemia(CLI) • Rest Pain • Tissue Loss • Gangrene Rest Pain/ Dependent Rubor Tissue Loss Gangrene Peripheral Artery Disease Diagnosis Segmental Pressures • Lower extremity Blood Pressures ABI • Nl >1.0, Diabetes will give elevated reading -

Peripheral Arterial Disease
SEMINAR Seminar Peripheral arterial disease Kenneth Ouriel Lower extremity peripheral arterial disease (PAD) most frequently presents with pain during ambulation, which is known as “intermittent claudication”. Some relief of symptoms is possible with exercise, pharmacotherapy, and cessation of smoking. The risk of limb-loss is overshadowed by the risk of mortality from coexistent coronary artery and cerebrovascular atherosclerosis. Primary therapy should be directed at treating the generalised atherosclerotic process, managing lipids, blood sugar, and blood pressure. By contrast, the risk of limb-loss becomes substantial when there is pain at rest, ischaemic ulceration, or gangrene. Interventions such as balloon angioplasty, stenting, and surgical revascularisation should be considered in these patients with so-called “critical limb ischaemia”. The choice of the intervention is dependent on the anatomy of the stenotic or occlusive lesion; percutaneous interventions are appropriate when the lesion is focal and short but longer lesions must be treated with surgical revascularisation to achieve acceptable long-term outcome. Peripheral arterial disease (PAD) comprises those entities ankle systolic pressure measured with a blood pressure at which result in obstruction to blood flow in the arteries, the malleolar level by the higher of the two brachial exclusive of the coronary and intracranial vessels. pressures. Defining PAD by an ankle-brachial index of Although the definition of PAD technically includes less than 0·95, a frequency of 6·9% was observed in problems within the extracranial carotid circulation, the patients aged 45–74 years, only 22% of whom had upper extremity arteries, and the mesenteric and renal symptoms.5 The frequency of intermittent claudication circulation, we will focus on chronic arterial occlusive increases dramatically with advancing age, ranging from disease in the arteries to the legs. -

Hypertension and Coronary Heart Disease
Journal of Human Hypertension (2002) 16 (Suppl 1), S61–S63 2002 Nature Publishing Group All rights reserved 0950-9240/02 $25.00 www.nature.com/jhh Hypertension and coronary heart disease E Escobar University of Chile, Santiago, Chile The association of hypertension and coronary heart atherosclerosis, damage of arterial territories other than disease is a frequent one. There are several patho- the coronary one, and of the extension and severity of physiologic mechanisms which link both diseases. coronary artery involvement. It is important to empha- Hypertension induces endothelial dysfunction, exacer- sise that complications and mortality of patients suffer- bates the atherosclerotic process and it contributes to ing a myocardial infarction are greater in hypertensive make the atherosclerotic plaque more unstable. Left patients. Treatment should be aimed to achieve optimal ventricular hypertrophy, which is the usual complication values of blood pressure, and all the strategies to treat of hypertension, promotes a decrease of ‘coronary coronary heart disease should be considered on an indi- reserve’ and increases myocardial oxygen demand, vidual basis. both mechanisms contributing to myocardial ischaemia. Journal of Human Hypertension (2002) 16 (Suppl 1), S61– From a clinical point of view hypertensive patients S63. DOI: 10.1038/sj/jhh/1001345 should have a complete evaluation of risk factors for Keywords: hypertension; hypertrophy; coronary heart disease There is a strong and frequent association between arterial hypertension.8 Hypertension is frequently arterial hypertension and coronary heart disease associated to metabolic disorders, such as insulin (CHD). In the PROCAM study, in men between 40 resistance with hyperinsulinaemia and dyslipidae- and 66 years of age, the prevalence of hypertension mia, which are additional risk factors of atheroscler- in patients who had a myocardial infarction was osis.9 14/1000 men in a follow-up of 4 years. -

Peripheral Arterial Disease
Peripheral arterial disease (Poor blood supply) Information sheet What is it? Peripheral arterial disease (PAD) is the narrowing of one or more arteries (blood vessels). It affects arteries that take blood to the legs, reducing the oxygen that gets to the foot that helps keep the tissues healthy. Also known as 'peripheral vascular disease' and sometimes called 'hardening of the arteries'. What causes it? The narrowing of the arteries is caused by atheroma. Atheroma is like fatty patches or 'plaques' that develop inside the lining of arteries. A patch of atheroma starts quite small, and causes no problems at first. Over the years it can thicken up and start to affect the blood flow through the arteries. (It is a bit like limescale that forms on the inside of water pipes). What are the symptoms? The typical symptom is like a ‘cramping’ sensation in the calves when walking a short distance. It is called 'intermittent claudication'. The pain is relieved when you stop walking. In more serious cases, cramp can be felt in the calf muscles during rest and at night. How can I help prevent it? The best way to help prevent this is to: Stop smoking Exercise regularly Maintain a healthy weight Eat a healthy diet Limit the amount of alcohol you drink (Contact your practice nurse for any further advice on the above) Take care of your feet www.oxleas.nhs.uk How do I take care of my feet? Try not to injure your feet as this can lead to an ulcer or infection developing more easily if the blood supply to the feet is reduced. -

Left Atheroma Mass and Occurrence Out-Of-Office Hypertension in an Extensive Population Raimondo Thomas*
Editorial iMedPub Journals Journal of Cardiovascular Medicine and Therapy 2021 www.imedpub.com Vol.4 No.1:e001 Left Atheroma Mass and Occurrence Out-of-Office Hypertension in an Extensive Population Raimondo Thomas* Department of Cardiology, Alfaisal University, Riyadh, Saudi Arabia *Corresponding author: Thomas R, Department of Cardiology, Alfaisal University, Riyadh, Saudi Arabia, E-mail: [email protected] Received date: February 01, 2021; Accepted date: February 15, 2021; Published date: February 22, 2021 Citation: Thomas R (2021) Left Atheroma Mass and Occurrence out- - of Office Hypertension in an Extensive Population. J Cardiovasc Med Ther Vol.4 No.1: e001 supplementation, physical activity, reduced alcohol consumption, and low-fat diets rich in fruits and vegetables have Abstract been effective in lowering BP and avert hypertension. Hypertension is an important risk factor for the development of cardiovascular disease, and is a major cause Discussion of morbidity and mortality worldwide. Traditionally, The current array of drug and nondrug therapeutic options hypertension diagnosis and treatment and clinical permit for control of hypertension to currently recommended evaluations of antihypertensive efficacy have been based on office blood pressure (BP) measurements; however, there is goal BP levels in all but the rarest patient and supply the increasing evidence that office measures may provide capacity to decrease BP to levels much lower than current inadequate or misleading estimates of a patient’s true BP guidelines recommend. Despite this capability, the vast majority status and level of cardiovascular risk. The introduction, and of patients with hypertension worldwide are untreated or badly endorsement by treatment guidelines, of 24-hour treated. -

Diabetic Ulcer Identification & Treatment
Diabetic Ulcer Identification & Treatment Bill Richlen PT, WCC, DWC, Clinical Instructor Wound Care Education Institute The information in this handout reflects the state of knowledge, current at time of publication; however, the authors do not take responsibility for data, information, and significant findings related to the topics discussed that became known to the general public following publication. The recommendations contained herein may not be appropriate for use in all circumstances. Decisions to adopt any particular recommendation must be made by the practitioner in light of available resources and circumstances presented by individual patients. Acknowledgements: This handout and presentation was prepared using information generally acknowledged to be consistent with current industry standards. The authors whose works are cited in the Bibliography Section of this manual are hereby recognized and appreciated. All product names, logos and trademarks used in this presentation are the property of the respective trademark owners. ® and ™ denote registered trademarks in the United States and other countries. "The use of NPUAP/EPUAP/PPPIA material does not imply endorsement of products or programs associated with the use of the material." Wound Care Education Institute (WCEI) Phone: 877-462-9234 Email: [email protected] www.wcei.net Copyright © 2018 by Wound Care Education Institute All Rights Reserved. WCEI grants permission for photocopying for limited personal or educational use only. This consent does not extend to other kinds of copying, such as copying for general distribution, for advertising or promotional purposes, for creating new collective works, or for resale. Treating Chronic Diabetic Wounds Outline I. Chronic Wounds A. Some Details 1. Definition: A wound that has failed to proceed orderly through the healing process or fails to result in durable closure.3 a.