Sub-Alpine Amphibian Distributions Related to Species Palatability to Non
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

9691.Ch01.Pdf
© 2006 UC Regents Buy this book University of California Press, one of the most distinguished univer- sity presses in the United States, enriches lives around the world by advancing scholarship in the humanities, social sciences, and natural sciences. Its activities are supported by the UC Press Foundation and by philanthropic contributions from individuals and institutions. For more information, visit www.ucpress.edu. University of California Press Berkeley and Los Angeles, California University of California Press, Ltd. London, England © 2006 by The Regents of the University of California Library of Congress Cataloging-in-Publication Data Sawyer, John O., 1939– Northwest California : a natural history / John O. Sawyer. p. cm. Includes bibliographical references and index. ISBN 0-520-23286-0 (cloth : alk. paper) 1. Natural history—California, Northern I. Title. QH105.C2S29 2006 508.794—dc22 2005034485 Manufactured in the United States of America 15 14 13 12 11 10 09 08 07 06 10987654321 The paper used in this publication meets the minimum require- ments of ansi/niso z/39.48-1992 (r 1997) (Permanence of Paper).∞ The Klamath Land of Mountains and Canyons The Klamath Mountains are the home of one of the most exceptional temperate coniferous forest regions in the world. The area’s rich plant and animal life draws naturalists from all over the world. Outdoor enthusiasts enjoy its rugged mountains, its many lakes, its wildernesses, and its wild rivers. Geologists come here to refine the theory of plate tectonics. Yet, the Klamath Mountains are one of the least-known parts of the state. The region’s complex pattern of mountains and rivers creates a bewil- dering set of landscapes. -

DAY HIKES Klamath National Forest a Stock Trail Though the Route Is Quite Steep Until the Leave No Trace Ridge Is Reached
DAY HIKES Klamath National Forest a stock trail though the route is quite steep until the Leave No Trace ridge is reached. Several beautiful meadows are Natural areas are fragile and we need your help to passed along the way before the junction with the protect and maintain them for future visitors to enjoy. Haypress Trail. The crossing of Let'er Buck Meadows Leave any area looking like you've never been there. can be confusing. From either direction pick out the Stay on designated trails to reduce erosion and opening in the trees almost straight across before scarring problems. When hiking in a trailless area, starting into the meadow. avoid paths that disturb streambanks, lakeshores, meadows, or wildlife. The wilderness belongs to you. Lovers Camp Trailhead Please help protect this special place by practicing low T43N R11W S8 impact traveling. Marble Valley - This moderately difficult hike of about 4.5 miles along the Canyon Creek Trail leads to Marble Mountain Wilderness Marble Valley at the PCT. Hikers can see historic Marble Valley Cabin built in the 1930's by the Forest Boulder Creek Trailhead Service for administrative work and staffed until the T43N R11W S32 1960's. From here, hikers can wander around the east slopes of Marble Mountain and the Marble Rim. Wrights Lakes - A very steep, 4.5 mile hike leads Remember to practice Leave No Trace hiking. from Boulder Creek Trailhead to the Wrights Lakes. Portions of this trail pass through meadow areas and Marble Rim - From Marble Valley, hike about 1 mile can be quite obscure. -

Cascades Frog Conservation Assessment
D E E P R A U R T LT MENT OF AGRICU United States Department of Agriculture Forest Service Pacific Southwest Research Station Cascades Frog General Technical Report PSW-GTR-244 Conservation Assessment March 2014 Karen Pope, Catherine Brown, Marc Hayes, Gregory Green, and Diane Macfarlane The U.S. Department of Agriculture (USDA) prohibits discrimination against its customers, employees, and applicants for employment on the bases of race, color, national origin, age, disability, sex, gender identity, religion, reprisal, and where applicable, political beliefs, marital status, familial or parental status, sexual orientation, or all or part of an individual’s income is derived from any public assistance program, or protected genetic information in employment or in any program or activity conducted or funded by the Department. (Not all prohibited bases will apply to all programs and/or employment activities.) If you wish to file an employment complaint, you must contact your agency’s EEO Counselor (PDF) within 45 days of the date of the alleged discriminatory act, event, or in the case of a personnel action. Additional information can be found online at http://www.ascr.usda.gov/complaint_filing_file.html. If you wish to file a Civil Rights program complaint of discrimination, complete the USDA Program Discrimination Complaint Form (PDF), found online at http://www.ascr.usda.gov/complaint_filing_cust. html, or at any USDA office, or call (866) 632-9992 to request the form. You may also write a letter containing all of the information requested in the form. Send your completed complaint form or letter to us by mail at U.S. -

Whitebark Pine Pilot Fieldwork Report Klamath National Forest
Whitebark Pine Pilot Fieldwork Report Klamath National Forest By Michael Kauffmann1, Sara Taylor2, Kendra Sikes3, and Julie Evens4 In collaboration with: Marla Knight, Forest Botanist, Klamath National Forest Diane Ikeda, Regional Botanist, Pacific Southwest Region, USDA Forest Service January, 2014 November 2018 Update: Page 47 1. Kauffmann, Michael E., Humboldt State University, Redwood Science Project, 1 Harpst Street, Arcata, CA 95521, [email protected] 2. Taylor, Sara M., California Native Plant Society, 2707 K Street, Suite 1, Sacramento, CA 95816, [email protected] 3. Sikes, Kendra., California Native Plant Society, 2707 K Street, Suite 1, Sacramento, CA 95816, [email protected] 4. Evens, Julie., California Native Plant Society, 2707 K Street, Suite 1, Sacramento, CA 95816, [email protected] Photo on cover page: Pinus albicaulis seen from Boulder Peak in the Marble Mountain Wilderness area, Klamath National Forest All photos by Michael Kauffmann unless otherwise noted All figures by Kendra Sikes unless otherwise noted Acknowledgements: We would like to acknowledge Marla Knight, Julie K. Nelson and Diane Ikeda for reviewing and providing feedback on this report. We also would like to thank Matt Bokach, Becky Estes, Jonathan Nesmith, Nathan Stephenson, Pete Figura, Cynthia Snyder, Danny Cluck, Marc Meyer, Silvia Haultain, Deems Burton and Peggy Moore for providing field data points or mapped whitebark pine for this project. Special thanks to Dr. Jeffrey Kane and Jay Smith for adventuring into the northern California wilds and helping with field work. Suggested report citation: Kauffmann, M., S. Taylor, K. Sikes, and J. Evens. 2014. Klamath National Forest: Whitebark Pine Pilot Fieldwork Report. Unpublished report. -

Russian Wilderness - Near Waterdog Lake B
RAIL T AL T L I O A O N F C G I E Russian Wilderness - Near Waterdog Lake B 3 s 6 ie 0 c e m p ile r s 41°15'53.68"N -122°58'48.18"W s x ife Elevation: 7,059' 3 on 2 c North South Finding the site: From the junction with the Deacon Rock: granite Fire evidence: no Percent cover: 20% Lee Trail, just at the edge of the wilderness, the trail climbs mature conifer, 5% regenerating conifer, 10% shrub, 1% slightly to a ridge which is the divide between the South herbaceous, 1% nonvascular Fork Salmon River and Russian Creek, whcih drains to the Height class: tree (15-20m), shrub (0.5-1m), herbaceous North Fork Salmon River. e spot is the obvious high (<0.5m) Vegetation Alliance: Abies magnica ssp. shastensis point along the trail and a great place to rest and take a few Select species within the plot pictures before a swim in Waterdog Lake. Trees: • Shasta fir Abies( magnica var. shastensis) About the site: As of summer 2016 the site is quite • White fir Abies( concolor) healthy with extensive conifer and shrub regeneration • Subalpine fir Abies( lasiocarpa) evident. Understory plants include ocean spray and pine- • Douglas-fir(Pseudotsuga menzeisii) mat manzanita. Of note is the one subapline r in the plot. • Mountain hemlock (Tsuga mertensiana) Shrubs: Russian Peak: 8,199’ • Pinemat manzanita (Arctostaphylos nevadensis) • Greenleaf manzanita (Arctostaphylos patula) • Tobacco brush (Ceanothus velutinus) • Bush chinquapin (Chrysolepis sempervirens) • Small-leaf creambush (Holodiscus discolor) Pacic Crest Trail • Bitter cherry(Prunus emarginata) • Whiteveined wintergreen (Pyrola picta) • One-sided Pyrola(Pyrola secunda) • Trailing gooseberry (Ribes binominatum) Herbaceous: • Feathery false lily of the valley (Maianthemum racemosum) • Buckwheat (Eriogonum sp.) • Mountain monardella (Monardella odoratissima) Waterdog Lake • Davis’ knotweed (Polygonum davisiae) When you visit.. -

Presented by the Salmon River Restoration Council Some of You May Be Thinking “So Where Is Salmon River?”
Salmon River Cooperative Noxious Weed Program (CNWP) Presented by the Salmon River Restoration Council Some of you may be thinking “So where is Salmon River?” Isn’t it in Idaho, Washington, or Oregon? The answer is: Northern California Salmon River Location 751 sq mi Salmon River Subbasin Godfrey Ranch where I’ve lived for 27 years My house has burned twice in forest fires Salmon/Klamath Confluence A E C W O I S L Y D S L T A E N M D S Katamin - “Center of the World” to the Karuk The Salmon River Wildlands Ecosystem North Fork from Etna Summit The Salmon River is one of the most biologically intact Subbasins in the west. It is the largest cold-water contributor to the Klamath River, and known as one of the cleanest rivers in the state. This 751 sq. mile watershed is entirely within the Klamath National Forest and is considered a key watershed by the Forest Service. Watershed analysis has been completed for the entire Subbasin, with the exception of Wooley Creek. The land base in the watershed includes: 98% Public Lands-USFS with 45% in wilderness, and 67% Karuk Ancestral Lands. Four communities lie widely dispersed within this watershed. There are approximately 250 year round and 100 part time residents in the subbasin. The Salmon River is documented as having an area in the Russian Wilderness that is one of the most diverse area for conifer species on Earth. It has long been known for its exceptionally high quality waters and is designated under the Wild and Scenic Act for the outstanding fisheries resources. -

Northwest California Wilderness, Recreation, and Working Forests Act
116TH CONGRESS REPORT " ! 2d Session HOUSE OF REPRESENTATIVES 116–389 NORTHWEST CALIFORNIA WILDERNESS, RECREATION, AND WORKING FORESTS ACT FEBRUARY 4, 2020.—Committed to the Committee of the Whole House on the State of the Union and ordered to be printed Mr. GRIJALVA, from the Committee on Natural Resources, submitted the following R E P O R T together with DISSENTING VIEWS [To accompany H.R. 2250] [Including cost estimate of the Congressional Budget Office] The Committee on Natural Resources, to whom was referred the bill (H.R. 2250) to provide for restoration, economic development, recreation, and conservation on Federal lands in Northern Cali- fornia, and for other purposes, having considered the same, report favorably thereon with an amendment and recommend that the bill as amended do pass. The amendment is as follows: Strike all after the enacting clause and insert the following: SECTION 1. SHORT TITLE; TABLE OF CONTENTS. (a) SHORT TITLE.—This Act may be cited as the ‘‘Northwest California Wilderness, Recreation, and Working Forests Act’’. (b) TABLE OF CONTENTS.—The table of contents for this Act is as follows: Sec. 1. Short title; table of contents. Sec. 2. Definitions. TITLE I—RESTORATION AND ECONOMIC DEVELOPMENT Sec. 101. South Fork Trinity-Mad River Restoration Area. Sec. 102. Redwood National and State Parks restoration. Sec. 103. California Public Lands Remediation Partnership. Sec. 104. Trinity Lake visitor center. Sec. 105. Del Norte County visitor center. Sec. 106. Management plans. Sec. 107. Study; partnerships related to overnight accommodations. TITLE II—RECREATION Sec. 201. Horse Mountain Special Management Area. 99–006 VerDate Sep 11 2014 08:14 Feb 07, 2020 Jkt 099006 PO 00000 Frm 00001 Fmt 6659 Sfmt 6631 E:\HR\OC\HR389.XXX HR389 2 Sec. -

Outreach Notice Klamath National Forest Salmon/Scott River Ranger District ______
USDA Forest Service Pacific Southwest Region _______________________________ Outreach Notice Klamath National Forest Salmon/Scott River Ranger District ___________________________________ DEPUTY DISTRICT RANGER, GS-0340-12 The Klamath National Forest is currently seeking a candidate for a Deputy District Ranger, GS-0340-12 located at the Salmon/Scott River Ranger District in Fort Jones, California. The purpose of this Outreach Notice is to inform prospective applicants of this upcoming opportunity. To express interest in this position, please complete the attached voluntary Outreach Interest Form and return to District Ranger Dave Hays at Hays, Russell D -FS [email protected] by close of business on March 20, 2013. DUTIES ASSOCIATED WITH THIS POSITION: As an alter-ego to the District Ranger, the incumbent assists in performing the full range of duties of a line officer. Examples include, but are not limited to, the following. Administers a complex Ranger District characterized by a number of significant multiple-use resource values in the areas of budget, human resources, administration, procurement, and resources. Participates with the District Ranger, the Forest Supervisor, and primary Forest and District staff in developing and organizing Forest and District policies and programs and other related concerns for management and protection of Forest resources. For more information on the position, please contact Dave Hays at 530-468-1235 or [email protected] ABOUT THE FOREST: The Klamath National Forest covers an area of 1,700,000 acres located in Siskiyou County in northern California and Jackson County in southern Oregon. The Forest is divided into two sections separated by the Shasta Valley and the Interstate 5 highway corridor. -

Bigfoot Trail • V3.2017 Map Set & Trail Description Michael Kauffmann Jason Barnes
The Bigfoot Trail • V3.2017 Map Set & Trail Description Michael Kauffmann Jason Barnes Bigfoot Trail Explore the biodiversity of the Klamath Mountains 32 conifer species, 360 miles, 6 wilderness areas, and 1 national park BACK COUNTRY PRESS Humboldt Co., CALIFORNIA Order this map set online at www.backcountrypress.com or www.bigfoottrail.org - limited edition print also available. © Copyright 2015 Backcountry Press - updated 4.26.2017 All rights reserved. No part of this publication may be reproduced, stored in retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise without written permission from the author. All photos and text by Michael Kauffmann Book layout and maps by Backcountry Press Cartography by Jason Barnes ( [email protected]) Consulting and GIS work by Justin Rohde Editing and trail notes by Sage Clegg (sageclegg.com) and Melissa Spencer GPX Coordinates by Sage Clegg Cover image: Jeffrey Kane hiking the BFT in the Red Buttes Wilderness Published by Backcountry Press | Kneeland, California ISBN 978-1-941624-04-3 BIGFOOT TRAIL - A WARNING The Bigfoot Trail is just a concept as of 2017. In essence it does not exist in the field and its name has been cho- sen to present the information contained in the website and this route description as a suggested way to travel, by foot, through the Klamath Mountains. The route is comprised of existing hiking trails and vehicle roads on public lands as a legal rights-of-way. Any person electing to follow the Bigfoot Trail does so voluntarily, yet ex- clusively, for the enjoyment of one of the most diverse and wild temperate coniferous forests on Earth. -
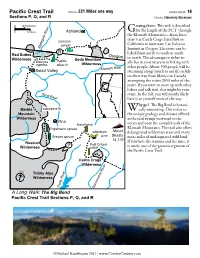
Pacific Crest Trail Distance: 221 Miles One Way Conifer Count: 19 Sections P, Q, and R Difficulty: Extremely Strenuous
Pacific Crest Trail Distance: 221 Miles one way Conifer Count: 19 Sections P, Q, and R Difficulty: Extremely Strenuous 0 10 Kilometers etting there: This trek is described r e v 0 10 Miles i Ashland for the length of the PCT through R G e the Klamath Mountains—from Inter- t a g state 5 at Castle Crags State Park in e common l p California to interstate 5 at Siskiyou p juniper A Summit in Oregon. The route can be R hiked from north to south or south Red Buttes Oregon Wilderness Soda Mountain to north. The advantage to either re- Siskiyou Pacific ally lies in your interests in hiking with cypress silver fir Wilderness r other people. About 300 people will be Klamath Rive Seiad Valley streaming along (south to north) in July on their way from Mexico to Canada attempting the entire 2650 miles of the route. If you want to meet up with other hikers and talk trail, this might be your Sc ott R route. In the fall, you will mostly likely iver have it to yourself most of the way. Q hy go? The Big Bend is botani- Marble subalpine fir Wcally interesting. This is due to Mountain the unique geology and climate offered Wilderness as the trail swings westward to the Etna foxtail pine ocean and onto the complex soils of the Engelmann spruce Klamath Mountains. The trail also offers whitebark Mount 6 designated wilderness areas and many Brewer spruce pine Shasta more miles of undesignated wild land. 14,179 If you have the stamina and the time, it Russian Port Orford- Wilderness P cedar is surely one of the greatest segments of the Pacific Crest Trail. -
Federal Register/Vol. 85, No. 207/Monday, October 26, 2020
67818 Federal Register / Vol. 85, No. 207 / Monday, October 26, 2020 / Proposed Rules ENVIRONMENTAL PROTECTION All submissions received must include 1. General Operation and Maintenance AGENCY the Docket ID No. for this rulemaking. 2. Biofouling Management Comments received may be posted 3. Oil Management 40 CFR Part 139 without change to https:// 4. Training and Education B. Discharges Incidental to the Normal [EPA–HQ–OW–2019–0482; FRL–10015–54– www.regulations.gov, including any Operation of a Vessel—Specific OW] personal information provided. For Standards detailed instructions on sending 1. Ballast Tanks RIN 2040–AF92 comments and additional information 2. Bilges on the rulemaking process, see the 3. Boilers Vessel Incidental Discharge National 4. Cathodic Protection Standards of Performance ‘‘General Information’’ heading of the SUPPLEMENTARY INFORMATION section of 5. Chain Lockers 6. Decks AGENCY: Environmental Protection this document. Out of an abundance of 7. Desalination and Purification Systems Agency (EPA). caution for members of the public and 8. Elevator Pits ACTION: Proposed rule. our staff, the EPA Docket Center and 9. Exhaust Gas Emission Control Systems Reading Room are closed to the public, 10. Fire Protection Equipment SUMMARY: The U.S. Environmental with limited exceptions, to reduce the 11. Gas Turbines Protection Agency (EPA) is publishing risk of transmitting COVID–19. Our 12. Graywater Systems for public comment a proposed rule Docket Center staff will continue to 13. Hulls and Associated Niche Areas under the Vessel Incidental Discharge provide remote customer service via 14. Inert Gas Systems Act that would establish national email, phone, and webform. We 15. Motor Gasoline and Compensating standards of performance for marine Systems encourage the public to submit 16. -

Table 7 - National Wilderness Areas by State
Table 7 - National Wilderness Areas by State * Unit is in two or more States ** Acres estimated pending final boundary development State National Wilderness Area Unit Name NFS Acreage Other Acreage Total Acreage Alabama Cheaha Wilderness Talladega National Forest 7,400 0 7,400 Dugger Mountain Wilderness Talladega National Forest 8,947 0 8,947 Sipsey Wilderness William B. Bankhead National Forest 25,770 83 25,853 Alabama Totals 42,118 83 42,200 2019 Land Areas Report Refresh Date: 10/19/2019 Table 7 - National Wilderness Areas by State * Unit is in two or more States ** Acres estimated pending final boundary development State National Wilderness Area Unit Name NFS Acreage Other Acreage Total Acreage Alaska Chuck River Wilderness Tongass National Forest 74,876 515 75,391 FS-administered, outside NFS bdy 0 5 5 Coronation Island Wilderness Tongass National Forest 19,118 0 19,118 Endicott River Wilderness Tongass National Forest 98,396 0 98,396 Karta River Wilderness Tongass National Forest 39,917 7 39,924 Kootznoowoo Wilderness Tongass National Forest 985,153 15,667 1,000,820 FS-administered, outside NFS bdy 0 654 654 Kuiu Wilderness Tongass National Forest 60,183 15 60,198 Maurille Islands Wilderness Tongass National Forest 4,814 0 4,814 Misty Fiords National Monument Wilderness Tongass National Forest 2,144,010 235 2,144,245 FS-administered, outside NFS bdy 0 15 15 Petersburg Creek-Duncan Salt Chuck Wilderness Tongass National Forest 46,758 0 46,758 Pleasant/Lemusurier/Inian Islands Wilderness Tongass National Forest 23,083 41 23,124 FS-administered,