Redalyc.Amphidromy in Shrimps: a Life Cycle Between Rivers and The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two Freshwater Shrimp Species of the Genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the Description of a New Species
A peer-reviewed open-access journal ZooKeys 923: 15–32 (2020) Caridina tetrazona 15 doi: 10.3897/zookeys.923.48593 RESEarcH articLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species Qing-Hua Chen1, Wen-Jian Chen2, Xiao-Zhuang Zheng2, Zhao-Liang Guo2 1 South China Institute of Environmental Sciences, Ministry of Ecology and Environment, Guangzhou 510520, Guangdong Province, China 2 Department of Animal Science, School of Life Science and Enginee- ring, Foshan University, Foshan 528231, Guangdong Province, China Corresponding author: Zhao-Liang Guo ([email protected]) Academic editor: I.S. Wehrtmann | Received 19 November 2019 | Accepted 7 February 2020 | Published 1 April 2020 http://zoobank.org/138A88CC-DF41-437A-BA1A-CB93E3E36D62 Citation: Chen Q-H, Chen W-J, Zheng X-Z, Guo Z-L (2020) Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species. ZooKeys 923: 15–32. https://doi.org/10.3897/zookeys.923.48593 Abstract A faunistic and ecological survey was conducted to document the diversity of freshwater atyid shrimps of Dawanshan Island. Two species of Caridina that occur on this island were documented and discussed. One of these, Caridina tetrazona sp. nov. is described and illustrated as new to science. It can be easily distinguished from its congeners based on a combination of characters, which includes a short rostrum, the shape of the endopod of the male first pleopod, the segmental ratios of antennular peduncle and third maxilliped, the slender scaphocerite, and the absence of a median projection on the posterior margin. -

A Genetical and Ecological Diversity of Fresh Water Prawns Macrobrachium Canarae and Caridina Gracilirostris from Kanyakumari Dist., Tamil Nadu, India
International Journal of Genetic Engineering and Biotechnology. ISSN 0974-3073 Volume 2, Number 1 (2011), pp. 23-32 © International Research Publication House http://www.irphouse.com A Genetical and Ecological Diversity of Fresh Water Prawns Macrobrachium Canarae and Caridina Gracilirostris from Kanyakumari Dist., Tamil Nadu, India Siva Ranjanee S.1 and Mariapan N.2 1Department of Biotechnology, Vels University, Pallavaram, Chennai, India 2Senior Medical Writer, SIRO Clinpharm Pvt. Ltd., Thane (W) 400 607. India E-mail: [email protected], [email protected] Abstract Fresh water prawns are cultured widely around the world but little is known about the levels and patterns of genetic diversity. This paper reports the RAPD analysis of two species of fresh water prawns of Atydiae and Palaemonidiae family and the genus Macrobrachium and Caridina collected from Kanyakumari district. Specimens are identified using species-specific morphological characteristics. Morphological characters have limitations of describing intra specific genetic diversity as they are polygenic and expressions can be modified by the environment. The advent of molecular technique made possible not only the genetic analysis and also the study of evolutionary relationship. Molecular analysis of the morphologically identified species are done by extracting the DNA from the animal and concentration of DNA is measured using Nanodrop spectrophotometer at a wavelength of 280nm. Further analysis was performed with RAPD (Rapid Amplified Polymorphic DNA) a PCR (Polymerase Chain Reaction) based technique. It consist of genomic DNA, amplified with randomly constructed oligonucleotides. Specific quantity of extracted DNA was then amplified by multiplex PCR using random primers and 16S rRNA gene products was then analyzed using a bioanalyzer. -

Effects of Drought and Hurricane Disturbances on Headwater Distributions of Palaemonid River Shrimp (Macrobrachium Spp.) in the Luquillo Mountains, Puerto Rico
J. N. Am. Benthol. Soc., 2006, 25(1):99–107 Ó 2006 by The North American Benthological Society Effects of drought and hurricane disturbances on headwater distributions of palaemonid river shrimp (Macrobrachium spp.) in the Luquillo Mountains, Puerto Rico Alan P. Covich1 Institute of Ecology, University of Georgia, Athens, Georgia 30602-2202 USA 2 3 Todd A. Crowl AND Tamara Heartsill-Scalley Ecology Center and Department of Aquatic, Watershed, and Earth Sciences, Utah State University, Logan, Utah 84322 USA Abstract. Extreme events (hurricanes, floods, and droughts) can influence upstream migration of macroinvertebrates and wash out benthic communities, thereby locally altering food webs and species interactions. We sampled palaemonid river shrimp (Macrobrachium spp.), dominant consumers in headwaters of the Luquillo Mountains of northeastern Puerto Rico, to determine their distributions along an elevational gradient (274–456 m asl) during a series of disturbances (Hurricane Hugo in 1989, a drought in 1994, and Hurricane Georges in 1998) that occurred over a 15-y period (1988À2002). We measured shrimp abundance 3 to 6 times/y in Quebrada Prieta in the Espiritu Santo drainage as part of the Luquillo Long- Term Ecological Research Program. In general, Macrobrachium abundance declined with elevation during most years. The lowest mean abundance of Macrobrachium occurred during the 1994 drought, the driest year in 28 y of record in the Espiritu Santo drainage. Macrobrachium increased in abundance for 6 y following the 1994 drought. In contrast, hurricanes and storm flows had relatively little effect on Macrobrachium abundance. Key words: dispersal, drainage networks, geomorphology, habitat preference, omnivores, prey refugia. Flow-based events often modify the important roles (Covich et al. -

A Migratory Shrimp's Perspective on Habitat Fragmentation in The
A MIGRATORY SHRIMP’S PERSPECTIVE ON HABITAT FRAGMENTATION IN THE NEOTROPICS: EXTENDING OUR KNOWLEDGE FROM PUERTO RICO BY MARCIA N. SNYDER1,3), ELIZABETH P. ANDERSON2,4) and CATHERINE M. PRINGLE1,5) 1) Odum School of Ecology, University of Georgia, Athens, Georgia 30602, U.S.A. 2) Global Water for Sustainability Program, Florida International University, Miami, FL 33199, U.S.A. ABSTRACT Migratory freshwater fauna depend on longitudinal connectivity of rivers throughout their life cycles. Amphidromous shrimps spend their adult life in freshwater but their larvae develop into juveniles in salt water. River fragmentation resulting from pollution, land use change, damming and water withdrawals can impede dispersal and colonization of larval shrimps. Here we review current knowledge of river fragmentation effects on freshwater amphidromous shrimp in the Neotropics, with a focus on Puerto Rico and Costa Rica. In Puerto Rico, many studies have contributed to our knowledge of the natural history and ecological role of migratory neotropical shrimps, whereas in Costa Rica, studies of freshwater migratory shrimp have just begun. Here we examine research findings from Puerto Rico and the applicability of those findings to continental Costa Rica. Puerto Rico has a relatively large number of existing dams and water withdrawals, which have heavily fragmented rivers. The effects of fragmentation on migratory shrimps’ distribution have been documented on the landscape-scale in Puerto Rico. Over the last decade, dams for hydropower production have been constructed on rivers throughout Costa Rica. In both countries, large dams restrict shrimps from riverine habitat in central highland regions; in Puerto Rico 27% of stream kilometers are upstream of large dams while in Costa Rica 10% of stream kilometers are upstream of dams. -

Los Camarones Del Bosque Nacional El Yunque
i te fijas bien en los ríos y las pozas Pueden nadar rápidamente hacia atrás por cortos Y...¿Cómo se reproducen los camarones ? de El Yunque puedes que veas algo periódos de tiempo haciendo un rápido moverse rápidamente por el fondo. movimiento o flexión en el abdomen. con el rabo. La reproducción y etapas de la vida de un Lo más probable hayas visto una de camarón son muy inetersantes. La época Slas 10 especies de camarones que habitan en Las etapas de la vida de un camarón son muy reproductiva de los camarones ocurre durante las aguas del interesantes. El camarón adulto vive en charcas y los meses de verano aunque hay especies que se bosque. La mayoría rápidos y por la noche se concentran sobre reproducen durante todo el año. de los sistemas de piedras, ramas de árboles y hojas pues es un agua dulce y animal de hábitos nocturnos. El apareamiento se lleva a cabo cuando el salobre de Puerto macho se coloca en ángulo recto con la hembra Rico están Micratya poeyi Especies y Hábitos alimenticios y le transfiere un espermatóforo a un habitados por receptáculo en el abdomen de la hembra. De 6 a camarones de las familias Atyidae y En Puerto Rico existen 9 especies de la familia 20 horas después del apareamiento, la hembra Palaemonidae. Atyidae y de éstas seis 6 se encuentran en los ríos carga bajo su abdomen una gran cantidad de de El Yunque. huevos. Esta cantidad depende de la especie y Estas curiosas especies de animales tienen Los miembros de el individuo. -

Molecular Evidence for Sequential Colonization and Taxon Cycling In
Molecular Ecology (2008) 17, 1066–1075 doi: 10.1111/j.1365-294X.2007.03637.x MolecularBlackwell Publishing Ltd evidence for sequential colonization and taxon cycling in freshwater decapod shrimps on a Caribbean island BENJAMIN D. COOK,* CATHERINE M. PRINGLE† and JANE M. HUGHES* *Australian Rivers Institute, Griffith University, Nathan, Queensland, Australia, †Institute of Ecology, University of Georgia, Athens, GA, USA Abstract Taxon cycling, i.e. sequential phases of expansions and contractions in species’ distributions associated with ecological or morphological shifts, are postulated to characterize dynamic biogeographic histories in various island faunas. The Caribbean freshwater shrimp assemblage is mostly widespread and sympatric throughout the region, although one species (Atyidae: Atya lanipes) is geographically restricted and ecologically and morphologically differentiated from other Atya species. Using patterns of nucleotide variation at the COI mtDNA gene in five species of freshwater shrimp (A. lanipes, A. scabra, A. innocuous; Xiphocarididae: Xiphocaris elongata; Palaemonidae: Macrobrachium faustinum) from Puerto Rico, we expected to detect a signature of sequential colonization in these shrimp, consistent with the concept of taxon cycling, and expected that A. lanipes would be at a different taxon stage (i.e. an early stage species) to all other species. We also examined patterns of genetic population structure in each species expected with poor, intermediate and well-developed abilities for among-river dispersal. Population expansions were detected in all species, although the relative timing of the expansions varied among them. Assuming that population expansions followed colonization of Puerto Rico by freshwater shrimp, results bear the hallmarks of sequential colonization and taxon cycling in this fauna. A. -

Caridina Variabilirostris (Crustacea: Decapoda: Atyidae), a New Species
Caridina variabilirostris (Crustacea: Decapoda: Atyidae), a new species of freshwater shrimp from Pohnpei (Micronesia) Valentin de Mazancourt, Gerard Marquet, Philippe Keith To cite this version: Valentin de Mazancourt, Gerard Marquet, Philippe Keith. Caridina variabilirostris (Crustacea: De- capoda: Atyidae), a new species of freshwater shrimp from Pohnpei (Micronesia). European Journal of Taxonomy, Consortium of European Natural History Museums, 2018, pp.1-16. 10.5852/ejt.2018.453. hal-02171169 HAL Id: hal-02171169 https://hal.archives-ouvertes.fr/hal-02171169 Submitted on 2 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. European Journal of Taxonomy 453: 1–16 ISSN 2118-9773 https://doi.org/10.5852/ejt.2018.453 www.europeanjournaloftaxonomy.eu 2018 · Mazancourt V. de et al. This work is licensed under a Creative Commons Attribution 3.0 License. Research article urn:lsid:zoobank.org:pub:445C8252-5FA2-49AA-AB89-E1C2DDEBEE7F Caridina variabilirostris (Crustacea: Decapoda: Atyidae), a new species of freshwater shrimp from Pohnpei (Micronesia) Valentin de MAZANCOURT 1,*, Gerard MARQUET 2 & Philippe KEITH 3 1,2,3 Muséum national d’Histoire naturelle, Département Adaptations du Vivant, UMR 7208, CP026, 57, rue Cuvier, 75231 Paris, Cedex 05, France. -

Caridina Wild Type Final 1
FRESHWATER SHRIMP WILD TYPE BY CHRIS LUKHAUP Paracaridina meridionalis During the course of evolution, Caridina logemanni They are also the most widely spread Paracaridina meridionalis freshwater dwarf shrimp have spread shrimp in the aquarium hobby. from the estuaries of larger rivers to However, recent research has found rapidly-flowing creeks in the that this genus is in urgent need of mountains,to enormous lakes, the a scientific review and re-structuring smallest trickles of water and even to as there are many discrepancies to cave waters. They are found from be found. The genus Neocaridina tropic to temperate climates today, has up until now been represented however, they have not conquered by 30 species, and has also found wide distribution in the hobby. Paracaridina meridionalis the arctic regions yet. The abundance Paracaridina meridionalis of different habitats has resulted in a Within the Genus Paracaridina just great variability in shrimp species and very few species have been described in stunning forms. Their sometimes but nevertheless some of them are truly impressive colours and patterns found in the shrimpkeeping hobby are the result of their adaptation to the different living conditions in their habitats. Enjoy and keep on shrimping ! With over 290 species, shrimp of the Paracaridina meridionalis genus Caridina are one of the most Paracaridina meridionalis diverse groups within the Atyidae family. Paracaridina meridionalis Paracaridina meridionalis Paracaridina meridionalis Paracaridina meridionalis Paracaridina meridionalis Caridina logemanni Caridina sp. “Vietnam” Caridina serrata Caridina serrata Caridina serrata Caridina trifasciata Caridina trifasciata Caridina trifasciata Caridina trifasciata Caridina sumatrensis paraCaridina sp. Caridina breviata Atyaephyra desmaresti Caridina simoni Caridina gracilirostris caridina serratirostris caridina serratirostris caridina serratirostris caridina serratirostris caridina sp” .Vietnam Blue” Caridina sp. -

Universidade De São Paulo Ffclrp
UNIVERSIDADE DE SÃO PAULO FFCLRP - DEPARTAMENTO DE BIOLOGIA PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA COMPARADA Avaliação sistemática de camarões de água doce do gênero Atya Leach, 1816 (Crustacea: Decapoda: Atyidae) por meio de dados moleculares Caio Martins Cruz Alves de Oliveira Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da USP, como parte das exigências para a obtenção do título de Mestre em Ciências, Área: BIOLOGIA COMPARADA Ribeirão Preto - SP 2017 UNIVERSIDADE DE SÃO PAULO FFCLRP - DEPARTAMENTO DE BIOLOGIA PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA COMPARADA Avaliação sistemática de camarões de água doce do gênero Atya Leach, 1816 (Crustacea: Decapoda: Atyidae) por meio de dados moleculares Caio Martins Cruz Alves de Oliveira Orientador: Prof. Dr. Fernando Luis Medina Mantelatto Co-orientadora: Profa. Dra. Mariana Terossi Rodrigues Mariano Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da USP, como parte das exigências para a obtenção do título de Mestre em Ciências, Área: BIOLOGIA COMPARADA Versão Original Ribeirão Preto - SP 2017 Autorizo a reprodução e divulgação total ou parcial deste trabalho, por qualquer meio convencional ou eletrônico, para fins de estudo e pesquisa, desde que citada a fonte. Oliveira, C. M. C. A. “Avaliação sistemática de camarões de água doce do gênero Atya Leach, 1816 (Crustacea: Decapoda: Atyidae) por meio de dados moleculares” Ribeirão Preto, 2017 vii+107p. Dissertação (Mestrado – Programa de Pós-graduação em Ciências. Área de concentração: Biologia Comparada). Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo (FFCLRP-USP). Orientador: Mantelatto, F.L.M.; Co-orientadora: Mariano, M.T.R. -
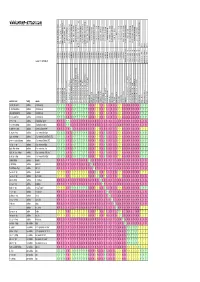
Crossbreeding/Interbreeding Chart Gotten from Web-Site
www.shrimp-attack.com nn blue blue pearl rib bee line ninja shrimp ninja green shrimp orchid shrimp yellow shrimp yellow sakura shrimp tuepfel shrimp orange shrimp yamato shrimp cardinal shrimp red tiger shrimp new new bee shrimp new bee shrimp new new bee shrimp new bee shrimp snowball shrimp snowball goldflake shrimp harlequin shrimp harlequin shrimp blue blue tiger shrimp red cherry shrimp red shrimp hawaii black tiger shrimp crystal red shrimp panda taiwan Bee bumble beebumble shrimp indian zebraindian shrimp crystal black shrimp blue blue bolt taiwan bee red ruby Taiwan bee Towuti/Matano Tiger king king kong Taiwan bee marmoreal dwarfshrimp crystal white bee shrimp golden golden chrystal red shrimp spec. spec. spec. cardina cardina cardina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina neocaridina neocaridina neocaridina neocaridina neocaridina neocaridina version 1.1. / 2010-04-20 halocaridina paracaridina paracaridina paracaridina common name family species cf.cf. cantonensiscantonensis 'Golden 'Golden CRS' CRS' cf.cf. cantonensiscantonensis redred tiger tiger cf.cf. cantonensiscantonensis sp.sp. 'bee' 'bee' cf.cf. cantonensiscantonensis sp.sp. 'white'white bee' bee' cf.cf. cantonensiscantonensis blackblack tiger tiger dennerli glaubrechti holthuisi maculata meridionalis multidentata propinqua serrata 'tuepfel' serratirostris spinata spongicola striata tenuirostris tumida venusta woltereckae rubra cf.cf. var. var.zhangjiajiensis zhangjiajiensis 'blue' 'blue' cf.cf. var. var.zhangjiajiensis zhangjiajiensis 'white' 'white' heteropodaheteropoda var.var. 'red' 'red' heteropodaheteropoda var.var. 'sakura' 'sakura' heteropodaheteropoda var.var. 'yellow' 'yellow' palmata blue bee princess bee larry shrimp cantonensis sp. cantonensis sp. -

Lines of Evidence for the Validity of Caridina Buehleri Roux (Crustacea : Decapoda : Atyidae) and for C
When molecules and morphology work together: lines of evidence for the validity of Caridina buehleri Roux (Crustacea : Decapoda : Atyidae) and for C. gueryi Marquet, Keith & Kalfatak as its junior synonym Valentin de Mazancourt, Gerard Marquet, Werner Klotz, Philippe Keith, Magalie Castelin To cite this version: Valentin de Mazancourt, Gerard Marquet, Werner Klotz, Philippe Keith, Magalie Castelin. When molecules and morphology work together: lines of evidence for the validity of Caridina buehleri Roux (Crustacea : Decapoda : Atyidae) and for C. gueryi Marquet, Keith & Kalfatak as its junior synonym. Invertebrate Systematics, CSIRO Publishing, 2017, 31 (2), pp.220. 10.1071/IS16044. hal-02171153 HAL Id: hal-02171153 https://hal.archives-ouvertes.fr/hal-02171153 Submitted on 2 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 When molecules and morphology work together: lines of evidence for the validity of 2 Caridina buehleri Roux, 1934 (Crustacea: Decapoda: Atyidae) and for C. gueryi Marquet, 3 Keith & Kalfatak, 2009 as its junior synonym. 4 VALENTIN de MAZANCOURT1*, GERARD MARQUET1, WERNER KLOTZ3, PHILIPPE 5 KEITH1 AND MAGALIE CASTELIN1-2 6 7 1 Muséum national d’Histoire naturelle, DMPA, UMR 7208, CP026, 57, rue Cuvier, F-75231 8 Paris, Cedex 05 9 2 Aquatic Animal Health Section, Fisheries and Oceans Canada, Pacific Biological Station, 10 3190 Hammond Bay Road, Nanaimo, BC, Canada V9T 6N7 11 3 Wiesenweg 1, A-6063 Rum, Austria. -

FOUR NEW PHILIPPINE SPECIES of FRESH-WATER the Description Of
Philippine Journal of Science, vol. 70, Bo. k December, 1939 D FOUR NEW PHILIPPINE SPECIES OF FRESH-WATER Ui SHRIMPS OF THE GENUS CARIDINA Q £ By GUILLERMO J. BLANCO Of the Division of Fisheries, Department of Agriculture and Commerce Manila THREE PLATES The description of these apparently new species of shrimps is based upon material collected from Laoag River, Laoag, Ilocos Norte Province, December 31, 1938, by Mr. Eulogio J. Martinez, and from a mountain stream, Helosig, Leyte, 1,500 feet above sea level, May 23, 1937, by Messrs. Dioscoro S. Rabor and M. Celestino, both of the Division of Fisheries, Bureau of Science. Genus CARIDINA Milne-Edwards Caridina MILNE-EDWARDS, Histoire Naturelle des Crustaces 2 (1837). C ARID IN A VILLADOLIDI sp. nov. Plate 1, figs. 1 to 9. Rostrum straight, saberlike, nearly reaching level of antennal scale; upper edge without teeth; lower edge with 6 teeth on distal half of its total length; a pair of setae posterior of rostrum (Plate 1, fig. 1). Antennal spine sharp; anteroinferior angle of carapace sharp-pointed. Eyes normal. Antennular pedun- cle reaching beyond tip of spine of antennular peduncle scale; basal segment not reaching beyond half of length of rostrum; second segment long, twice its width. Antennal scale slender, three times as long as broad. Mandible with three spines of incisor process and setse (Plate 1, fig. 2). Terminal joint of third maxilliped (Plate 1, fig. 3) with eight spines and nu- merous setse. Carpus of first perseopod (Plate 1, fig. 4) twice as long as distal breadth, excavation at distal end slightly cres- cent-shaped.