WO 2017/044387 Al 16 March 2017 (16.03.2017) P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global and International Studies Satisfy Requirements for Both the Major and Minor in Global and International Studies
The following list is a comprehensive survey of undergraduate courses offered at IUPUI that Global and International Studies satisfy requirements for both the major and minor in Global and International Studies. Courses Course Offerings List are grouped by general requirements, modern language requirements, regional/area concen- Summer 2016 trations, and thematic concentrations. • Students may take up to 6 credit hours of I415 Independent Study, but no more than 3 credits in any specific concentration. • Courses that are cross-listed under more than one regional and/or thematic concentration may only be counted towards one. • Classes marked with an * are variable subtitle offerings, courses listed under the same number that cover distinct subjects (such as Anthropology A460). Your transcript will indicate a different title each time the course is completed. Students may therefore take multiple courses listed under the same number, but can only count that course toward the regional or thematic concentration under which it’s listed here. • If students have questions about a course offered that is not on this list, please contact Dr. Michael Snodgrass ([email protected]) or Penny Saltsman ([email protected]). The list will be updated periodically as new courses are added, dropped, or cancelled due to circumstances beyond the control of Global & International Studies. • NOTE: Highlighted courses are being offered during the indicated semester (for the most up-to-date information, search for courses in SIS). FRENCH (FREN) International Studies Courses World Languages Courses F131 First Year French I INTL I100 Intro to International Students who began the program prior to Fall 2013 must F132 First Year French II satisfy second-year proficiency in a modern foreign F203 Second Year French I Studies language. -

Camp Brochure
RESORT CATALOGUE Therefore everyone who hears these words of mine and puts them into practice is like a wise man who built his house on the rock. Matthew 7:24 OUR RESORTS Thank you for considering one of the UCSA Resorts for your next Christian getaway. We have eleven unique venues settled in some spectacular locations, provided by God. We look forward to being a part of enriching the journey of folks with Christ at one of these super spots. Should you require any further information, please feel free to make contact with one of our representatives at the resort you may be interested in (indicated on each page at the bottom). Bainskloof Pg 2 Waterval Pg 8 Western Cape Western Cape Die Hoekie Pg 3 Kleinmond Pg 9 Gauteng Western Cape Groot-Brakrivier Pg 4 Retief Camp Pg 10 Western Cape Free State Jeffreys Bay Pg 5 Uitsig Pg 11 Eastern Cape Western Cape Winklespruit Pg 6 Karmel Pg 12 KwaZulu-Natal Western Cape Kijk in de Pot Pg 7 Western Cape Accommodation Accommodation units: 13 Beds: 134 (120 in dormitories and 14 in two self-catering units) Nearby The town of Wellington Outdoor activities • swimming (only allowed under supervision of a first-aider) • hiking (centre’s own scenic eco-hiking route; Bainskloof hiking in the adjacent Western Cape Limietberg Nature Reserve requires a permit from Overview Cape Nature) Aptly referred to as the “mountain camp”, UCSA’s Bainskloof camp centre high up in the Limietberg Mountains combines first-rate accommodation and facilities with an unsurpassed Facilities and services experience in nature. -

Cap Classique Route D E F R a FRANSCHHOEK PASS to VILLIERSDORP & HERMANUS MORENA FRANSCHHOEK
® V K I G E N O E H R O H N S C S N cap classique route D E F R A FRANSCHHOEK PASS TO VILLIERSDORP & HERMANUS MORENA FRANSCHHOEK MOUNTAINS L HAUTE CABRIÈREA M STONY BROOK VERDUN B GKRANS R A E C COLMANT CAP H IDD EY MY WYN T M CLASSIQUE & LLCHAMPAGNE HUGUENOT VA E N X EE MONUMENT CE R DIEU DONNÉ T EXCELSIOR LSIO G OD UITKYK N A R VINEYARDS E N I U E G L U H RO H U BER G TS O VL WEMMERSHOEK EI MOUNTAINS FRANSCHHOEK VILLAGE & SURROUNDS K E O H LA PRO H ASSENBERG C D S VENCE N A R HAWEQWA F GRANDE PROVENCE O NATURE RESERVE T HERITAGE EWINEI ESTATE N L I SV BERT D O A R O R RICKETY BRIDGE WINERY N I A LEOPARD’S LEAP M WEMMERSHOEK FAMILY VINEYARDS BERG RIVER DAM DAM H LA MOTTE AP PY VA L L E Y MÔRESON TOPIARY WINES GROOT DRAKENSTEIN MOUNTAINS DRAKENSTEIN ANTHONIJ RUPERT WINES PRISON MANDELA STATUE FRANSCHHOEK MOTOR MUSEUM GROOT DRAKENSTEIN GAMES CLUB FRANSCHHOEK VALLEY PEARL VALLEY GOLF ESTATE BOSCHENDAL WINES ALLÉE BLEUE ESTATE & WINES TO PAARL & N1 TO (EXIT 59) PLAISIR DE MERLE STELLENBOSCH R301 R310 N1 TO WORCESTER D MÉTHODE CAP CLASSIQUE A TO PAARL & N1 O Méthode Cap Classique (MCC) is the South R45 R EXIT 55 M NOBLE HILL African term used to describe a sparkling wine U I D N made following the classic French method of N1 O IM champagne making. The fine bubbles in each -S S BACKSBERG T U bottle areSIMONSBERG produced by inducing a secondary M ESTATE CELLAR NATURE RESERVE P R101 A fermentation in the bottle. -
Nuwedrif Exit for Wellington Turn-Off for Franschhoek from Cape Town from Worcester from Stellenbosch 4-WAY STOP EXIT 47 EXIT 59
GPS: -33.669444, 18.971944 // 33°40’10”S and 18°58’19”E BAINSKLOOF PASS approx 45mins - 1Hour from Cape Town Route A (R44 ) Tweede Tol Nature Camp Route B (R45) approx 45mins - 1Hour from Cape Town - No trucks allowed on this route Wit Krom ASS BAINSKLOOF P M A L M E S B U R Y Bain R Limietberg O A Monument D Nature R44 Reserve Most Southerly Blockhouse berg Hex N O O RD AG TE R P AA R303 RL BAINSKLOOF PASS RO Wellington AD 4-WAY STOP Historic Church Wit pange St Cham R44 / R45 4-Way Stop Nelsons Creek Le Bac Berg Diemersfontein Newtown Country Estate Windmeul Winery & Cellar R303 N Nuwedrif R45 Spruit 0 1 2 3 4 5 km Wellington Farm Malmesbury 0 1 2 3 miles Paarlberg Gearbox Rotia Kafee R45 Dal Josafat Dal Josafat DU TOITSKLOOF P AN RIEBEECKSports Ground V LANG JAN R312 Orleans Caravan Park R301 ASS MEAKER WESTHOVEN OSCH Molenaars PAARL MOUNTAIN OOSB SONSTRAAL MAIN Ebenhaëzer Manor House TUNN Huguenot GUENOT EL Bethel PAARL HU Dam Millwater LADY GREY KLEIN DRAKENSTEIN R101 Wild Flower Reserve 1 From Worcester MARKET LANGENHOVEN To avoid Toll Road, take this Scenic Bridge turn-o for Du Toitskloof Pass Nantes T Paarl Rock Boland Park EXIT 62 D Dam V JAN RIEBEECK L B Cricket Stadium R45 R V E AN Prisoner of War Cross V I R To avoid Toll Road, take this Ceramic Studio G R E (Clementina van der Walt) turn-o for Du Toitskloof Pass B Victoria Dam MAIN R44 P EXIT 59 a lm Ampitheatre i ARBORETUM e Afrikaans Language TAILLEFER t T Paarl S Monument S Nic Taylor’s Nut Farm A Paarl T M A B Golf D A Course Exit for Wellington Diamant EXIT 55 W Entertainment E M M Centre E R K S R101 H O L E K Animal House of Olives Turn-off for Franschhoek E A M O Zone Berg River Holiday Resort I U 1 Drakenstein R45 N N T Lion Park A EXIT 47 D I N Butterfly World R S Klapmuts A OAD Cillie K IUM R OND D E SIM Le Bonheur R EN N From Cape Town Crocodile Farm OR R301 S SIMONSVLEI. -

Social Assistance in South Africa: Its Potential Impact on Poverty
Social assistance in South Africa: Its potential impact on poverty. Submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy in Development Studies at the Institute for Social Development, University of the Western Cape Supervisors: Prof. P. le Roux and Prof. R. Botman (Full text version but printed in smaller fonts) written by: Claudia Haarmann e-mail: [email protected] date: 30.08.2000 Acknowledgments II Acknowledgments I would like to use this opportunity to thank my promoters Pieter le Roux and Russel Botman for their extraordinary help, support and encouraging friendship during the whole process of this research. Many thanks to COSATU for initiating this research and for the debate about a comprehensive social security system in South Africa. Special thanks to the members of the reference group for their com- mitment: Oupa Bodibe, Kenneth Creamer, Neil Coleman, Sandy Liebenberg, Neva Makgetla, Ravi Naidoo, Viviene Taylor and Eddie Webster. The discussions, inputs and invaluable comments at the workshops were key to the success of this project. Thanks to Conrad Barberton, Jacqui Boulle, Kenneth Creamer, Tania Flood, Ute and Hanns Hedrich- Lessing, Sandy Liebenberg, Johann Magerman, and Alison Tilley. First of all for their very special friendship, but also for many critical discussions on the topic which were so essential for the research. Thanks to Murray Leibbrandt and James Midgley for making time to discuss the research and for their highly appreciated comments. To the chairperson of the Portfolio Committee on Welfare, Cassim Saloojee: Thanks for the friend- ship, trust and encouragement throughout the process. -

The Great Green Outdoors
R ive r S w r a r ive t r r R ivi ve e i p r e R i 01 WITZANDS AQUIFER NATURE RESERVE D Cape Town is the world’s #1 water-saving city. S p w e i a D Please keep using water wisely. r t MAMRE r i Protecting the Atlantis aquifer and the THE GREAT GREEN OUTDOORS vi e aquifer re-charge areas, it is the main r FOR MORE VISIT C APETOWN.GOV.ZA / THINKWATER water supply for the Atlantis, Mamre FOLLOW @CITYOFCT ON FACEBOOK AND TWITTER Sustaining Cape Town’s Water Supply GOUDA and Pella communities. The reserve FOR MORE VISIT C APETOWN.GOV.ZA / THINKWATER has impressive sand dunes and views of Table Mountain. Add on a visit to FOLLOW @CITYOFCT ON FACEBOOK AND TWITTER the quaint mission village of Mamre, ATLANTIS RIEBEEK VOELVLEI DAM KASTEEL Only flush when Take short, stop- Don’t leave the established in the 17th century. The Cape Town is a water-scarce city that is diversifying its sources of water, but it you really need to. start showers. tap running while original water mill has been restored brushing teeth. still gets most of its water from rain-fed dams. The catchment areas feeding our WATER WOLSELEY and is used as a museum today. dams are relatively pristine, but need to be preserved. The alien invasive plants REPORTING in the catchments suck up water before it can get to our dams, and there are HERMON Help preserve our precious water resources. D WITZANDS i e To report burst pipes, faulty p R SILWERSTROOMSTRAND AQUIFER ive programmes to remove them to increase the yield of water to the Western Cape r Use alternative water safely. -

La Paris Estate
LA PARIS ESTATE • WEDDING PACKAGES • AUGUST 2018 - JULY 2020 YOUR WEDDING AT LA PARIS ESTATE Thank you for your interest in La Paris Estate and for allowing us the opportunity to provide you and your guests with an unforgettable experience. La Paris Estate is situated just 8km’s outside the historical town of Franschhoek and offers a variety of venues and romantic outdoor settings to cater for weddings of all sizes and styles. The breath-taking mountains, perfectly manicured lawns, vibrant gardens and magnificent Oak trees provide a dream like environment for you and your partner to pledge your devotion to one another. VENUE THE WINERY The Winery has a historical and glamorous atmosphere. Inside rests a beautiful black & white checkered marble floor beneath intricate hand forged brass lanterns which were rescued from a ruined french chateau. This venue is suitable for wedding ceremonies or receptions and welcomes up to 120 guests to celebrate an elegant Royal occasion. THE LYCEUM The Lyceum provides a muted canvas with its raw brick, dramatic arch windows and glass doors. The beautiful crystal chandeliers which reflect on the white marbled floors, add a touch of elegance. Tendrils of fragrance pour into the Lyceum from the beautiful white flower garden. This venue welcomes up to 400 guests for a standard banquet but can accommodate more depending on requirements. THE ARBOUR This idyllic outside area with its wrought Iron gazebo surrounded by tall luscious hedges with grass underfoot and the mountain standing guard provides the perfect place for your ceremony. THE ROSE GARDEN The Rose Garden provides a magical location for an intimate wedding ceremony filled with romance. -

General Household Survey, 2018 STATISTICS SOUTH AFRICA I P0318
STATISTICAL RELEASE P0318 General Household Survey 2018 Embargoed until: 28 May 2019 11:00 ENQUIRIES: FORTHCOMING ISSUE: EXPECTED RELEASE DATE User Information Services GHS 2019 May 2020 Tel.: (012) 310 8600 www.statssa.gov.za [email protected] T +27 12 310 8911 F +27 12 310 8500 Private Bag X44, Pretoria, 0001, South Africa ISIbalo House, Koch Street, Salvokop, Pretoria, 0002 STATISTICS SOUTH AFRICA i P0318 CONTENTS LIST OF FIGURES ........................................................................................................................................... iv LIST OF TABLES ............................................................................................................................................. vi Abbreviations ................................................................................................................................................... vii Summary and Key Findings ........................................................................................................................... viii 1 Introduction ........................................................................................................................................... 1 1.1 Purpose ................................................................................................................................................. 1 1.2 Survey scope ........................................................................................................................................ 1 2 Basic population statistics -

Here Guests Can Enjoy the Beautiful Views of the Surrounding Mountains
THANK YOU FOR YOUR ENQUIRY Congratulations on your engagement and thank you for considering Freedom Hill Family Vineyards as your wedding venue. We would love to be part of your special day and we hope to be able to make it unforgettable. With a picturesque view that overlooks the Simonsberg Mountains, Freedom Hill Winery is sure to give a truly impeccable ambiance creating the feeling of pure bliss and joyful freedom. We look forward to hosting you and your wedding guests and will do our utmost to ensure that you have a distinct, excellent experience. R301, WEMMERSHOEK RO AD, PAARL, 7646 PO BOX 6353 • UNIEDA L , 7 6 1 2 T: 021-886 6895 • F: 021-882 8207• E: [email protected] W: www.freedomhill.co.za (REG NR 1998/03121/07) BTW: 4480172370 PACKAGE CONTENTS: The Venues Venue Rates Venue Fee Includes Venue Fee Excludes Terms & Conditions Directions THE VENUE The venue has a neutral, fresh finish that can be decorated as desired. A large front door opens from the venue to a spacious terrace and a widely spread lawn where guests can enjoy the beautiful views of the surrounding mountains. The venue also includes a beautiful bar area that can be used for extra seating or as a dance floor. The venue can accommodate the following: Up to 80 seated guests with a dance floor in the bar area or on the terrace. Up to 120 guests for a cocktail style function. THE AMPHITHEATRE The amphitheatre located on the lawn outside the venue has a picturesque view of the Simonsberg Mountains and can be used as the wedding ceremony area. -

Paarl A3 Tearoff
Malmesbury Hermon Malmesbury SCALE: 1km N 19 PAARDEBERG SCALE: 2km RETIEF N MOLL 24 RIVE IN D UNTA S MO ILLIP 2 R44 JAN PH 28 SKOOL AYAMA GROENBERG 5 1 KERK Berg River Krom River Krom River 47 10 PEARL AARD OOR-P EBERG MOUNTAIN LANG V Bainskloof Pass BO LANG R44 R301 RIPARIA E 6 19 V O L G R45 WELLINGTON X O 8 F R44 DU LOT PERDEBERG 16 37 Spruit River 13 13 3 KONING WESTHOVEN 4319 WINDMEUL Berg River WIJNSKOOL 18 OOSBOSCH WINDMEUL 21 6 32 ALPHOREX BOSCH RIDGEBACK UNDER OAKS 8 27 ST 59 AL JOSAFAT HOR R44 52 46 D OPTEN 25 22 R45 11 44 RHEBOKSKLOOF 40 R45 Berg River DRUK-MY-NIET 15 BOLAND CELLAR 9 2 24 DU TOITS KLOOF 39 MOUNTAINS 56 SANDRIFT PEARL MOUNTAIN R312 4 3815 5 1 MALAN 14 1 17 OLYVEN R45 BOTHA PLEIN BLACK PEARL WINES 24 NEDERBURG N DRAKENST 16 KLEI EIN 57 HOSPITAL 46 20 49 BO MEUL 2 20 OLSEN WATERKANT LOUIE DORP Palmiet River 10 R44 4 17 41 MILL 12 R101 14 MELLASAT EXIT Worcester 30 62 T 42 Berg River NANTES PAARL 12 Huguenot 7 25 LADY GREY MOUNTAIN Toll Plaza 6 BO-LADY GREY BREDA D NEW D-AGTER PAA GRANDE ROCHE 45 9 Wildeperdejag River R I R SU L N1 KLOOF A 16 LOOP V Durbanville 20 36 E THE MASON’S WINERY VENDÔME 60 L 20 LANDSKROON EXIT VICTORIA VAN DER LINGEN U 27 59 3 O KWV KWV WINE B 1 9 2 SENSORIUM E EMPORIUM L 11 TEXTILE 4 L 2 SPICE ROUTE R E DURR LABORIE I E 7 5 EILAND R V 22 26 4 I 22 PAINTED WOLF A WINES 15 P 13 R FAIRVIEW G 3 N R O HOUT N E ZANDWIJK B EXIT 8 55 Berg River MARKET 58 N1 R45 14 18 15 AURET DEVINE R101 11 FRANSCHHOEK R301 MOUNTAINS HAWKSMOOR WINES EXIT PONTAC 47 7 13 11 PATRIOT 29 54 28 10 Cape Town 17 MONSBERG-PAAR -

Contents CONTENTS
Back to contents CONTENTS LOCATION INTRODUCTION CONFERENCING DAY CONFERENCE PACKAGES VENUE SET-UP CONFERENCE ROOM SPECIFICATIONS FLOOR PLANS SITE MAP ACCOMMODATION FACILITIES SPA & GOLF ACTIVITIES 2 3 R44 R312 R27 SOUTHERN PAARL PAARL FARMS R301 M14 N1 M48 R304 R45 N7 N1 R44 BLOUBERGSTRAND M13 DURBANVILLE R101 R301 RIETVLEI HAWEQWA NATURE RESERVE LOCATION BRACKENFELL R45 R101 M23 R304 CAPE R45 SIMONSBERG N1 TOWN BRACKENFELL SOUTH NATURE RESERVE CITY OF BELLVILLE PAROW M23 CLOETESVILLE M6 R102 FRANSCHHOEK GPS: -34.3172,19.1347 N1 M5 BERG RIVER DAM R43 STELLENBOSCH BANHOEK CONSERVANCY M62 BELHAR R300 KUILS RIVER THREE WATERS N2 DELFT NATURE RESERVE M12 JONKERSHOEK Cape Town 100 km M6 NATURE RESERVE SOUTHERN SUBURBS GUGULETU R45 VILLIERSDORP BLUE DOWN Cape Town International Airport 70 km R44 PHILLIPI Kleinmond 8 km M36 KHAYELITSHA MITCHELLS PLAIN HELDERBERG HOTTENTOTS-HOLLAND Hermanus 22 km NATURE RESERVE HOUT BAY N2 TOKAI FOREST M6 LOURENS RIVIER R321 SOMERSET WEST R43 STRAND DIRECTIONS: M4 GROENLANDBERG NATURE RESERVE FISH HOEK Take the N2 from Cape Town heading towards Somerset West and continue until you reach Sir Lowry’s Pass. Continue KOMMETJIE M6 GORDONS BAY GRABOUW over this pass and then turn left onto the R43 (to Hermanus). Continue on this road for approximately 10 km until R44 N2 M65 M4 (Kleinmond sign) where you turn right. Follow the road for about 2 km until you see the Arabella Country Estate sign on N2 HOTTRNTONS-HOLLARD M66 MOUNTAIN CATCHMEN SIMON’S TOWN your left. R44 HOTTENTOTS-HOLLAND MOUNTAIN CATCHMENT AREA N2 Or take the spectacularly scenic coastal route from Gordon’s Bay (off the N2 from Cape Town), passing through Betty’s CALEDON M65 M4 Bay and Kleinmond. -

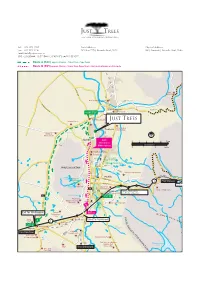
Franschhoek Artisan Food Route Map (PDF)
Image courtesy of Babylonstoren FRANSCHHOEK PASS TO VILLIERSDORP Franschhoek EXCELSIOR T Mountains BAGATELLE ROBERTSVLEI H WILHELMINA CABRIÈRE C Huguenot RE B Café BonBon Monument M VERDUN A A Shofar HUGUENOT FRANSCHHOEK VILLAGE A Methodist L Church 7 La Bourgogne Y Church DIRKIE UYS LE & SURROUNDS AL AKADEMIE V LAMBRECHTS 17 N BERG MIDDAGKRANS E Huguenot Monument RE 24 Tuk Tuk Microbrewery EXCELSIOR G DISA AALWYN 9 UITKYK EXCELSIOR ERICA De Villiers Chocolate Café D BERGPolice Station CABRIÈRE A UNION T N O United 25 I 12 The Franschhoek Olive Oil Company RESERVOIR N EL Congregational E H Church U DANIEL HUGO U G Post Office O R G O U B E H R KLIP T RESERVOIR VAN WIJK LA PROVENCE S V KLEIN L E CABRIÈRE I LA ROCHELLE CABRIÈRE DASSENBERG Town Hall 23 Terbodore Coffee Roasters VLEI RTS DE LA RAY DASSENBERG BE Franschhoek RO 13 Village Market 15 Huguenot Fine Chocolates Gooding’s FABRIEK Groves 14 BICCCS 4 KRUGER MARIA La Cotte Inn / 18 K E Fromages de France 11 Franschhoek O 20 Maison Berg River Dam H Medicinal H C S DIRKIE UYS AKADEMIELOUIS BOTHA Herb Garden N A La Motte R HA F P 19 PY O V Petrol Station T A Wine Estate LL N E Y I & Historic Walk D A HAUMANN HUGUENOT O LA COTTE R HUGUENOT N Verenigde I 6 Bread & Wine Gereformeerde A NAUDE Kerk DIRKIE UYS M DE WET AKADEMIE 10 DE VILLIERS UITKYK Cellar Groot Drakenstein The Franschhoek Mountains LA PROVENCE R45 TO SIMONDIUM & N1 (Main Road from Cape Town) Drakenstein 2 Anthonij Rupert Wyne Prison Mandela Statue Franschhoek 06 Motor Museum Groot Drakenstein Games Club FRANSCHHOEK Solms-Delta