Neuromorphic VLSI Designs for Spike Timing and Rate-Based Synaptic Plasticity with Application in Pattern Classification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How-To Gnome-Look Guide
HHOOWW--TTOO Written by David D Lowe GGNNOOMMEE--LLOOOOKK GGUUIIDDEE hen I first joined the harddisk, say, ~/Pictures/Wallpapers. right-clicking on your desktop Ubuntu community, I and selecting the appropriate You may have noticed that gnome- button (you know which one!). Wwas extremely look.org separates wallpapers into impressed with the amount of different categories, according to the customization Ubuntu had to size of the wallpaper in pixels. For Don't let acronyms intimidate offer. People posted impressive the best quality, you want this to you; you don't have to know screenshots, and mentioned the match your screen resolution. If you what the letters stand for to themes they were using. They don't know what your screen know what it is. Basically, GTK is soon led me to gnome-look.org, resolution is, click System > the system GNOME uses to the number one place for GNOME Preferences > Screen Resolution. display things like buttons and visual customization. The However, Ubuntu stretches controls. GNOME is Ubuntu's screenshots there looked just as wallpapers quite nicely if you picked default desktop environment. I impressive, but I was very the wrong size, so you needn't fret will only be dealing with GNOME confused as to what the headings about it. on the sidebar meant, and I had customization here--sorry no idea how to use the files I SVG is a special image format that Kubuntu and Xubuntu folks! downloaded. Hopefully, this guide doesn't use pixels; it uses shapes Gnome-look.org distinguishes will help you learn what I found called vectors, which means you can between two versions of GTK: out the slow way. -
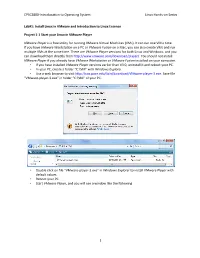
Install Linux in Vmware and Introduction to Linux Essence
CPSC2800‐Introducation to Operating System Linux Hands‐on Series Lab#1: Install Linux in VMware and Introduction to Linux Essence Project 1‐1 Start your Linux in VMware Player VMware Player is a free utility for running VMware Virtual Machines (VMs). It can run one VM a time. If you have VMware Workstation on a PC or VMware Fusion on a Mac, you can also create VMs and run multiple VMs at the same time. There are VMware Player versions for both Linux and Windows, and you can download them directly from http://www.vmware.com/ddownload/player/. You should not install VMware Player if you already have VMware Workstation or VMware Fusion installed on your computer. • If you have installed VMware Player versions earlier than V3.0, uninstall it and reboot your PC. • In your PC, create a folder “C:\VM” with Windows Expxplorer. • Use a web browser to visit http://csis.pace.edu/lixin/download/VVMware‐player‐3.exe. Save file “VMware‐player‐3.exe” in folder “C:\VM” of your PC. • Double click on file “VMware‐player‐3.exe” in Windoows Explorer to install VMware Player with default values. • Reboot your PC. • Start VMware Player, and you will see a window like the following. 1 CPSC2800‐Introducation to Operating System Linux Hands‐on Series • Click on menu item “File|Preferences…” 2 CPSC2800‐Introducation to Operating System Linux Hands‐on Series • In the “Preferences” window, uncheck for software updates, and click on the “Download Alll Components Now” button so you can later install VMware Tools in your new VMs without Internet access. -

Free As in Freedom
Daily Diet Free as in freedom ... • The freedom to run the program, for any purpose (freedom 0). Application Seen elsewhere Free Software Choices • The freedom to study how the program works, and adapt it to Text editor Wordpad Kate / Gedit/Vi/ Emacs your needs (freedom 1). Access to the source code is a precondition for this. Office Suite Microsoft Office KOffice / Open Office • The freedom to redistribute copies so you can help your Word Processor Microsoft Word Kword / Writer Presentation PowerPoint KPresenter / Impress neighbor (freedom 2). Spreadsheet Excel Kexl / Calc • The freedom to improve the program, and release your Mail & Info Manager Outlook Thunderbird / Evolution improvements to the public, so that the whole community benefits (freedom 3). Access to the source code is a Browser Safari, IE Konqueror / Firefox precondition for this. Chat client MSN, Yahoo, Gtalk, Kopete / Gaim IRC mIRC Xchat Non-Kernel parts = GNU (GNU is Not Unix) [gnu.org] Netmeeting Ekiga Kernel = Linux [kernel.org] PDF reader Acrobat Reader Kpdf / Xpdf/ Evince GNU Operating Syetem = GNU/Linux or GNU+Linux CD - burning Nero K3b / Gnome Toaster Distro – A flavor [distribution] of GNU/Linux os Music, video Winamp, Media XMMS, mplayer, xine, player rythmbox, totem Binaries ± Executable Terminal>shell>command line – interface to type in command Partition tool Partition Magic Gparted root – the superuser, administrator Graphics and Design Photoshop, GIMP, Image Magick & Corel Draw Karbon14,Skencil,MultiGIF The File system Animation Flash Splash Flash, f4l, Blender Complete list- linuxrsp.ru/win-lin-soft/table-eng.html, linuxeq.com/ Set up Broadband Ubuntu – set up- in terminal sudo pppoeconf. -

Technology User Guide Volume III: DRC INSIGHT
Technology User Guide Volume III: DRC INSIGHT WISCONSIN Data Recognition Corporation (DRC) 13490 Bass Lake Road Maple Grove, MN 55311 Wisconsin Service Line: 1-800-459-6530 DRC INSIGHT Portal: https://wi.drcedirect.com Email: [email protected] Revision Date: November 12, 2020 COPYRIGHT Copyright © 2020 Data Recognition Corporation The following items in DRC INSIGHT are protected by copyright law: • The User Guide. • All text and titles on the software’s entry and display, including the look and feel of the interaction of the windows, supporting menus, pop-up windows, and layout. DRC INSIGHT Online Learning System and DRC INSIGHT Portal are trademarked by Data Recognition Corporation. Any individuals or corporations who violate these copyrights and trademarks will be prosecuted under both criminal and civil laws, and any resulting products will be required to be withdrawn from the marketplace. The following are trademarks or registered trademarks of Microsoft Corporation in the United States and/or other countries: Internet Explorer Microsoft Windows Windows Vista Windows XP Windows 7 Windows 8 Windows 10 The following are trademarks or registered trademarks of Apple Corporation in the United States and/or other countries: Apple Macintosh Mac OS X and macOS iPad iPadOS iOS* *iOS is a trademark or registered trademark of Cisco in the U.S. and other countries and is used under license. Safari The following are trademarks or registered trademarks of Google Corporation in the United States and/or other countries. Chrome Chromebook Google Play The following is a trademark or registered trademark of Mozilla Corporation in the United States and/or other countries. -

Ubuntu® 1.4Inux Bible
Ubuntu® 1.4inux Bible William von Hagen 111c10,ITENNIAL. 18072 @WILEY 2007 •ICIOATENNIAl. Wiley Publishing, Inc. Acknowledgments xxi Introduction xxiii Part 1: Getting Started with Ubuntu Linux Chapter 1: The Ubuntu Linux Project 3 Background 4 Why Use Linux 4 What Is a Linux Distribution? 5 Introducing Ubuntu Linux 6 The Ubuntu Manifesto 7 Ubuntu Linux Release Schedule 8 Ubuntu Update and Maintenance Commitments 9 Ubuntu and the Debian Project 9 Why Choose Ubuntu? 10 Installation Requirements 11 Supported System Types 12 Hardware Requirements 12 Time Requirements 12 Ubuntu CDs 13 Support for Ubuntu Linux 14 Community Support and Information 14 Documentation 17 Commercial Support for Ubuntu Linux 18 Getting More Information About Ubuntu 19 Summary 20 Chapter 2: Installing Ubuntu 21 Getting a 64-bit or PPC Desktop CD 22 Booting the Desktop CD 22 Installing Ubuntu Linux from the Desktop CD 24 Booting Ubuntu Linux 33 Booting Ubuntu Linux an Dual-Boot Systems 33 The First Time You Boot Ubuntu Linux 34 Test-Driving Ubuntu Linux 34 Expioring the Desktop CD's Examples Folder 34 Accessing Your Hard Drive from the Desktop CD 36 Using Desktop CD Persistence 41 Copying Files to Other Machines Over a Network 43 Installing Windows Programs from the Desktop CD 43 Summary 45 ix Contents Chapter 3: Installing Ubuntu on Special-Purpose Systems 47 Overview of Dual-Boot Systems 48 Your Computer's Boot Process 48 Configuring a System for Dual-Booting 49 Repartitioning an Existing Disk 49 Getting a Different Install CD 58 Booting from a Server or Altemate -

LIFE Packages
LIFE packages Index Office automation Desktop Internet Server Web developpement Tele centers Emulation Health centers Graphics High Schools Utilities Teachers Multimedia Tertiary schools Programming Database Games Documentation Internet - Firefox - Browser - Epiphany - Nautilus - Ftp client - gFTP - Evolution - Mail client - Thunderbird - Internet messaging - Gaim - Gaim - IRC - XChat - Gaim - VoIP - Skype - Videomeeting - Gnome meeting - GnomeBittorent - P2P - aMule - Firefox - Download manager - d4x - Telnet - Telnet Web developpement - Quanta - Bluefish - HTML editor - Nvu - Any text editor - HTML galerie - Album - Web server - XAMPP - Collaborative publishing system - Spip Desktop - Gnome - Desktop - Kde - Xfce Graphics - Advanced image editor - The Gimp - KolourPaint - Simple image editor - gPaint - TuxPaint - CinePaint - Video editor - Kino - OpenOffice Draw - Vector vraphics editor - Inkscape - Dia - Diagram editor - Kivio - Electrical CAD - Electric - 3D modeller/render - Blender - CAD system - QCad Utilities - Calculator - gCalcTool - gEdit - gxEdit - Text editor - eMacs21 - Leafpad - Application finder - Xfce4-appfinder - Desktop search tool - Beagle - File explorer - Nautilus -Archive manager - File-Roller - Nautilus CD Burner - CD burner - K3B - GnomeBaker - Synaptic - System updates - apt-get - IPtables - Firewall - FireStarter - BackupPC - Backup - Amanda - gnome-terminal - Terminal - xTerm - xTerminal - Scanner - Xsane - Partition editor - gParted - Making image of disks - Partitimage - Mirroring over network - UDP Cast -

Install Gnome Software Center Arch
Install gnome software center arch Upstream URL: License(s): GPL2. Maintainers: Jan Steffens. Package Size: MB. Installed Size: Installed Size: MB. gnome-software will be available as a preview in It can install, remove applications on systems with PackageKit. It can install updates on Gnome software will not start / Applications & Desktop. A quick video on Gnome Software Center in Arch Linux. Gnome unstable repository. There is a component called Polkit that is used by many applications to request root permissions to do things (it can do so because it's a. GNOME Software on #archlinux with native PackageKit backend, and this is a gui for installing software, ala ubuntu software manager, but distro This is some kind of Ubuntu Software Centre, with comments and all that. Need help installing Gnome Software Center for Arch Linux? Here are some instructions: Click DOWNLOAD HERE in the menu. Download the file. Make the file. I had to install it with along with packagekit. This is what's missing to make Antergos *the* beginner-friendly Arch-based distro, or general So, it is not a bad idea for the “Gnome Software Center” to include by default. GNOME software software center graphic that we will find the default in future releases of Fedora in addition to being installed in Arch Linux Please help me to install GNOME Software on. GNOME Software Will Work On Arch Linux With PackageKit the Alpm/Pacman back-end for using this GNOME application to install and. From: Sriram Ramkrishna ; To: desktop-devel-list devel-list gnome org>; Subject: gnome- software/packagekit. -

ANJUTA – Um Breve Tutorial 1
ANJUTA – Um breve tutorial 1 Sumário Anjuta um breve tutorial..................................................................................................................2 Definição.....................................................................................................................................2 Recursos do Anjuta.....................................................................................................................3 Antes de começar........................................................................................................................4 O primeiro projeto...........................................................................................................................5 Incrementando o projeto............................................................................................................12 Invocando os poderes do Glade................................................................................................12 O esqueleto básico do programa...............................................................................................14 Qual comando usar para compilar?...........................................................................................16 Compilando e rodando o projeto...................................................................................................17 Dividir para conquistar!............................................................................................................17 Criando os novos arquivos...................................................................................................18 -

Tumor Necrosis Factor (TNF)-Receptor 1 and 2 Mediate Homeostatic Synaptic Plasticity of Denervated Mouse Dentate Granule Cells
www.nature.com/scientificreports OPEN Tumor necrosis factor (TNF)- receptor 1 and 2 mediate homeostatic synaptic plasticity of Received: 04 March 2015 Accepted: 06 July 2015 denervated mouse dentate granule Published: 06 August 2015 cells Denise Becker†, Thomas Deller & Andreas Vlachos Neurological diseases are often accompanied by neuronal cell death and subsequent deafferentation of connected brain regions. To study functional changes after denervation we generated entorhino- hippocampal slice cultures, transected the entorhinal pathway, and denervated dentate granule cells in vitro. Our previous work revealed that partially denervated neurons respond to the loss of input with a compensatory, i.e., homeostatic, increase in their excitatory synaptic strength. TNFα maintains this denervation-induced homeostatic strengthening of excitatory synapses. Here, we used pharmacological approaches and mouse genetics to assess the role of TNF-receptor 1 and 2 in lesion-induced excitatory synaptic strengthening. Our experiments disclose that both TNF-receptors are involved in the regulation of denervation-induced synaptic plasticity. In line with this result TNF-receptor 1 and 2 mRNA-levels were upregulated after deafferentation in vitro. These findings implicate TNF-receptor signaling cascades in the regulation of homeostatic plasticity of denervated networks and suggest an important role for TNFα-signaling in the course of neurological diseases accompanied by deafferentation. Homeostatic control of cortical excitability, connectivity and plasticity is considered essential for normal brain function1–3. Work from the past years has unraveled a wealth of information on compensatory mechanisms acting in the brain to keep the activity in neuronal networks within a physiological range4. Among the best studied mechanisms is homeostatic synaptic plasticity5–8, which adjusts synaptic strength to changes in network activity in a compensatory manner. -
Hello Everyone. I Introduce You Voyager GE 19.04 That Continues the Adventure with the Desktop Gnome Shell Version 3.32 by Introducing New Features
Hello everyone. I introduce you Voyager GE 19.04 that continues the adventure with the desktop Gnome Shell version 3.32 by introducing new features. With the promise finally realized to have a light Gnome system, fast, fluid and powerful. This version is based on the Linux 5.0 kernel and distribution Ubuntu Disco Dingo . 19.04 is an intermediate version upgrade nine months preparing the future version 20.04 LTS - Long-term Support - 5 years that happens every two years, when Voyager will be available in 2 versions, GNOME Shell and Xfce. Also you can choose according to your wishes and capacity machines. The general idea of Voyager is to introduce in Gnome Gnome Shell preinstalled extensions and scripts grouped in a box that optimize the system with a choice of necessary software. A video presentation and pictures are available. Soon for this new human and digital adventure. Voyager wants multi-profile and multi-task in an environment aesthetic and immersive as possible and that, from the origins of traveling, so that time spent on your machine is the most pleasant. In summary, the general idea is that for each profile, we have options available types that can activate or not. Voyager GE 19.04 based on Ubuntu 19.04 will cycle nine months of support and updates. For information, these are intermediate versions that prepare Future LTS. You can then seamlessly transition to 19.10 with automatic up-to-date system and so on until the next LTS. Voyager GE is not a distribution with its deposits but a customized variant of Ubuntu as mentioned above, with all its official repositories. -

AT&T Synaptic Compute As a Service
Version 1.5 June 2015 SM AT&T Synaptic Compute as a Service Instruction Guide to AT&T Operating System Usage © 2015 AT&T Intellectual Property. All rights reserved. AT&T and the AT&T logo are trademarks of AT&T Intellectual Property Notice Copyright 2015 © AT&T Intellectual Property. All rights reserved. AT&T, the AT&T logo and all other AT&T marks contained herein are trademarks of AT&T Intellectual Property and/or AT&T affiliated companies. All other marks contained herein are the property of their respective owners. This document is not an offer, commitment, representation or warranty by AT&T and is subject to change. The information contained in this document should not be duplicated, transmitted, or disclosed, in whole or in part without the expressed written consent of AT&T. Information in this document is subject to change without notice. AT&T assumes no responsibility for any errors or omissions in this document. Use of this document and the information is pursuant to the terms and conditions of your service agreement with AT&T. Windows is a trademark of the Microsoft® group of companies. Red Hat® Enterprise Linux® is a trademark of Red Hat, Inc. © 2015 AT&T Intellectual Property. All rights reserved. AT&T and the AT&T logo are trademarks of AT&T Intellectual Property. Contents 1.0 Overview ..................................................................................................................................................... 4 2.0 Creating New Virtual Machines ........................................................................................................... -

Release Notes for Debian 10 (Buster), IBM System Z
Release Notes for Debian 10 (buster), IBM System z The Debian Documentation Project (https://www.debian.org/doc/) September 30, 2021 Release Notes for Debian 10 (buster), IBM System z This document is free software; you can redistribute it and/or modify it under the terms of the GNU General Public License, version 2, as published by the Free Software Foundation. This program is distributed in the hope that it will be useful, but WITHOUT ANY WARRANTY; without even the implied warranty of MERCHANTABILITY or FITNESS FOR A PARTICULAR PURPOSE. See the GNU General Public License for more details. You should have received a copy of the GNU General Public License along with this program; if not, write to the Free Software Foundation, Inc., 51 Franklin Street, Fifth Floor, Boston, MA 02110-1301 USA. The license text can also be found at https://www.gnu.org/licenses/gpl-2.0.html and /usr/ share/common-licenses/GPL-2 on Debian systems. ii Contents 1 Introduction 1 1.1 Reporting bugs on this document . 1 1.2 Contributing upgrade reports . 1 1.3 Sources for this document . 2 2 What’s new in Debian 10 3 2.1 Supported architectures . 3 2.2 What’s new in the distribution? . 3 2.2.1 UEFI Secure Boot . 4 2.2.2 AppArmor enabled per default . 4 2.2.3 Optional hardening of APT . 5 2.2.4 Unattended-upgrades for stable point releases . 5 2.2.5 Substantially improved man pages for German speaking users . 5 2.2.6 Network filtering based on nftables framework by default .