Colour Perception Across the Species Birgitta Dresp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lepidoptera: Papilionoidea) Q ⇑ Marianne Espeland A,B, , Jason P.W
Molecular Phylogenetics and Evolution 93 (2015) 296–306 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Ancient Neotropical origin and recent recolonisation: Phylogeny, biogeography and diversification of the Riodinidae (Lepidoptera: Papilionoidea) q ⇑ Marianne Espeland a,b, , Jason P.W. Hall c, Philip J. DeVries d, David C. Lees e, Mark Cornwall a, Yu-Feng Hsu f, Li-Wei Wu g, Dana L. Campbell a,h, Gerard Talavera a,i,j, Roger Vila i, Shayla Salzman a, Sophie Ruehr k, David J. Lohman l, Naomi E. Pierce a a Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, USA b McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Powell Hall, 2315 Hull Road, Gainesville, FL 32611, USA c Department of Systematic Biology-Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560-127, USA d Department of Biological Sciences, University of New Orleans, 2000 Lake Shore Drive, New Orleans, LA 70148, USA e Department of Zoology, University of Cambridge, Cambridge CB2 3EJ, UK f Department of Life Science, National Taiwan Normal University, Taipei, Taiwan g The Experimental Forest, College of Bio-Resources and Agriculture, National Taiwan University, Nantou, Taiwan h Division of Biological Sciences, School of Science, Technology, Engineering & Mathematics, University of Washington Bothell, Box 358500, 18115 Campus Way NE, Bothell, WA 98011-8246, USA i Institut de Biologia Evolutiva (CSIC-UPF), Pg. Marítim de la Barceloneta 37, 08003 Barcelona, Spain j Faculty of Biology & Soil Science, St. -

Behavior of the Amazonian Damselfly Chalcopteryx Scintillans Mclachlan
International Journal of Odonatology, 2014 Vol. 17, No. 4, 251–258, http://dx.doi.org/10.1080/13887890.2014.983189 Behavior of the Amazonian damselfly Chalcopteryx scintillans McLachlan (Zygoptera: Polythoridae) and comments on its morphological distinction from C. rutilans (Rambur) Rhainer Guillermo-Ferreiraa,b∗, Ulisses Gaspar Neissc, Neusa Hamadad and Pitágoras C. Bispob aFaculdade de Ciências Biológicas e Ambientais, Universidade Federal da Grande Dourados/UFGD, Dourados, Mato Grosso do Sul, Brazil; bDepartamento de Ciências Biológicas, Faculdade de Ciências e Letras de Assis, Universidade Estadual Paulista/UNESP, Assis, São Paulo, Brazil; cInstituto de Natureza e Cultura - INC/BC, Universidade Federal do Amazonas/UFAM, Benjamin Constant, Amazonas, Brazil; d Instituto Nacional de Pesquisas da Amazônia/INPA, Coordenação de Biodiversidade/CBio, Manaus, Amazonas, Brazil (Received 26 June 2014; accepted 28 October 2014) Polythorid damselflies are Neotropical stream dwellers, whose behavior has rarely been recorded. Here we describe the territorial and courtship behavior of Chalcopteryx scintillans McLachlan, an Amazonian damselfly with shiny copper-colored hind wings. Territorial behavior consists of aerial contests, when males engage in threat displays and mutual pursuits in ascending and rocking flights. During courtship, males hold their coppery hind wings still while hovering with their forewings, showing the hind wings to females, which hover in front of the male in response. After copulation, the male exhibits the courtship flight again by hovering over the oviposition resource (i.e. fallen tree trunk) on the stream. The females oviposit on the trunk while the males guard them by perching near and hovering around them con- stantly. We also present behavioral notes on reproductive and oviposition behavior, and comments on the differentiation between C. -

Spatial Restriction of Light Adaptation Inactivation in Fly Photoreceptors and Mutation-Induced
The Journal of Neuroscience, April 1991, 7 f(4): 900-909 Spatial Restriction of Light Adaptation and Mutation-Induced Inactivation in Fly Photoreceptors Baruch Minke and Richard Payne” Department of Neuroscience, The Howard Hughes Medical Institute, The Johns Hopkins University School of Medicine, Baltimore, Maryland 21205 The spatial spread within fly photoreceptors of 2 forms of We describe simple preparations of the retinas of the housefly, desensitization by bright light have been investigated: the Musca domestica, and the sheepblowfly, Lucilia cuprina, that natural process of light adaptation in normal Musca photo- allow recording from individual ommatidia in vitro, using a receptors and a receptor-potential inactivation in the no- suction pipette similar to that used to record from rod photo- steady-state (nss) mutant of the sheep blowfly Lucilia. The receptors of the vertebrate retina (Baylor et al., 1979; for a suction-electrode method used for recording from verte- preliminary use of this method, see also Becker et al., 1987). brate rods was applied to fly ommatidia. A single ommatid- The suction-electrode method has 2 advantages when applied ium in vitro was partially sucked into a recording pipette. to fly photoreceptors. First, the method might prove to be more Illumination of the portion of the ommatidium within the pi- convenient than the use of intracellular microelectrodes when pette resulted in a flow of current having a wave form similar recording from small photoreceptors, such as those of Drosoph- to that of the receptor potential and polarity consistent with ila. Recordings from Drosophila are needed to perform func- current flow into the illuminated region of the photorecep- tional tests on mutant flies. -
Skin Patterning in Octopus Vulgaris and Its Importance for Camouflage
Skin patterning in Octopus vulgaris anditsimportance for camouflage DORSAL ANTERiOR POSTERIOR VENTRAL Wilma Meijer-Kuiper D490 Dqo "Scriptie"entitled: j Skin patterning in Octopus vulgaris and its importance for camouflage Author: Wilma Meijer-Kuiper. Supervisor: Dr. P.J. Geerlink. April, 1993. Cover figure from Wells, 1978. Department of Marine Biology, University of Groningen, Kerklaan 30, 9750 AA Haren, The Netherlands. TABLE OF CONTENTS PAGE Abstract 1 1. Introduction 2 1.1. Camouflage in marine invertebrates 2 1 .2. Cephalopods 2 2. General biology of the octopods 4 2.1. Octopus vulgar/s 4 3. Skin patterning in Octopus 6 3.1. Patterns 6 3.2. Components 8 3.3. Units 9 3.4. Elements 10 3.4.1. Chromatophores 10 3.4.2. Reflector cells and iridophores 16 3.4.3. Leucophores 18 4. The chromatic unit 20 5. Nervous system 22 5.1. Motor units 22 5.2. Electrical stimulation 22 5.3. Projection of nerves on the skin surface 24 5.4. The nervous control system 25 5.5. Neurotransmitters 27 6. Vision in cephalopods, particularly in O.vu!garis 28 6.1. Electro-physiological experiments 28 6.2. Photopigments and receptor cells 28 6.3. Behavioural (training) experiments 29 6.4. Eye movements and optomotor responses 29 7. Camouflage in a colour-blind animal 31 7.1. Matching methods 31 7.2. Countershading reflex 35 8. Conclusive remarks 36 Literature 38 ABSTRACT Camouflage is a method by which animals obtain concealment from other animals by blending in with their environment. Of the cephalopods, the octopods have an extra-ordinary ability to match their surroundings by changing the colour and texture of their skin. -

Nei Vision Report.Pdf
VISION RESEARCH A NATIONAL PLAN: 19992003 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE NATIONAL INSTITUTES OF HEALTH NATIONAL EYE INSTITUTE A Report of the National Advisory Eye Council DEDICATION Not long after the Roys great intellect, careful experimental approach, creation of the National Eye and keen scientific insights earned him the MERIT Institute (NEI) by Congress Award from the NEI and the Friedenwald Award from in 1968, a young, promising the Association for Research in Vision and vision research scientist Ophthalmology. While maintaining an active and named Roy Steinberg vigorous vision research program, Roy also found time received one of the first to serve as an adviser to the National Institutes of Roy H. Steinberg, M.D., Ph.D. NEI Research Career Health and the NEI. He was a member and later Chair Development Awards. This of the Visual Disorders Study Section, the forerunner marked the beginning of of todays Visual Sciences C. He served as Chair of a long and productive association between the NEI the Retinal Diseases Panel for the NEIs Vision and a researcher who served the vision community ResearchA National Plan: 1987 Evaluation and in many ways. Update and as a consultant to the 19781982 and the 19941998 national plans. He authored the highlights With his great breadth of knowledge and sharp mind, and recommendations from two NEI-sponsored Roy had a clearer grasp than most of the many facets workshopsthe first on the Cell Biology of Retinal of retinal research, both clinical and laboratory. Most Detachment in 1986, and the second on Repair and productive scientists establish a single theme to their Replacement to Restore Sight in 1991. -

Longwing (Heliconius) Butterflies Combine a Restricted Set of Pigmentary and Structural Coloration Mechanisms Bodo D
Wilts et al. BMC Evolutionary Biology (2017) 17:226 DOI 10.1186/s12862-017-1073-1 RESEARCH ARTICLE Open Access Longwing (Heliconius) butterflies combine a restricted set of pigmentary and structural coloration mechanisms Bodo D. Wilts1,2* , Aidan J. M. Vey1, Adriana D. Briscoe3 and Doekele G. Stavenga1 Abstract Background: Longwing butterflies, Heliconius sp., also called heliconians, are striking examples of diversity and mimicry in butterflies. Heliconians feature strongly colored patterns on their wings, arising from wing scales colored by pigments and/or nanostructures, which serve as an aposematic signal. Results: Here, we investigate the coloration mechanisms among several species of Heliconius by applying scanning electron microscopy, (micro)spectrophotometry, and imaging scatterometry. We identify seven kinds of colored scales within Heliconius whose coloration is derived from pigments, nanostructures or both. In yellow-, orange- and red-colored wing patches, both cover and ground scales contain wavelength-selective absorbing pigments, 3-OH-kynurenine, xanthommatin and/or dihydroxanthommatin. In blue wing patches, the cover scales are blue either due to interference of light in the thin-film lower lamina (e.g., H. doris) or in the multilayered lamellae in the scale ridges (so-called ridge reflectors, e.g., H. sara and H. erato); the underlying ground scales are black. In the white wing patches, both cover and ground scales are blue due to their thin-film lower lamina, but because they are stacked upon each other and at the wing substrate, a faint bluish to white color results. Lastly, green wing patches (H. doris) have cover scales with blue-reflecting thin films and short-wavelength absorbing 3-OH-kynurenine, together causing a green color. -

Bulletin Biological Assessment Boletín RAP Evaluación Biológica
Rapid Assessment Program Programa de Evaluación Rápida Evaluación Biológica Rápida de Chawi Grande, Comunidad Huaylipaya, Zongo, La Paz, Bolivia RAP Bulletin A Rapid Biological Assessment of of Biological Chawi Grande, Comunidad Huaylipaya, Assessment Zongo, La Paz, Bolivia Boletín RAP de Evaluación Editores/Editors Biológica Claudia F. Cortez F., Trond H. Larsen, Eduardo Forno y Juan Carlos Ledezma 70 Conservación Internacional Museo Nacional de Historia Natural Gobierno Autónomo Municipal de La Paz Rapid Assessment Program Programa de Evaluación Rápida Evaluación Biológica Rápida de Chawi Grande, Comunidad Huaylipaya, Zongo, La Paz, Bolivia RAP Bulletin A Rapid Biological Assessment of of Biological Chawi Grande, Comunidad Huaylipaya, Assessment Zongo, La Paz, Bolivia Boletín RAP de Evaluación Editores/Editors Biológica Claudia F. Cortez F., Trond H. Larsen, Eduardo Forno y Juan Carlos Ledezma 70 Conservación Internacional Museo Nacional de Historia Natural Gobierno Autónomo Municipal de La Paz The RAP Bulletin of Biological Assessment is published by: Conservation International 2011 Crystal Drive, Suite 500 Arlington, VA USA 22202 Tel: +1 703-341-2400 www.conservation.org Cover Photos: Trond H. Larsen (Chironius scurrulus). Editors: Claudia F. Cortez F., Trond H. Larsen, Eduardo Forno y Juan Carlos Ledezma Design: Jaime Fernando Mercado Murillo Map: Juan Carlos Ledezma y Veronica Castillo ISBN 978-1-948495-00-4 ©2018 Conservation International All rights reserved. Conservation International is a private, non-proft organization exempt from federal income tax under section 501c(3) of the Internal Revenue Code. The designations of geographical entities in this publication, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of Conservation International or its supporting organizations concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

The Genetics and Evolution of Iridescent Structural Colour in Heliconius Butterflies
The genetics and evolution of iridescent structural colour in Heliconius butterflies Melanie N. Brien A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy The University of Sheffield Faculty of Science Department of Animal & Plant Sciences Submission Date August 2019 1 2 Abstract The study of colouration has been essential in developing key concepts in evolutionary biology. The Heliconius butterflies are well-studied for their diverse aposematic and mimetic colour patterns, and these pigment colour patterns are largely controlled by a small number of homologous genes. Some Heliconius species also produce bright, highly reflective structural colours, but unlike pigment colour, little is known about the genetic basis of structural colouration in any species. In this thesis, I aim to explore the genetic basis of iridescent structural colour in two mimetic species, and investigate its adaptive function. Using experimental crosses between iridescent and non-iridescent subspecies of Heliconius erato and Heliconius melpomene, I show that iridescent colour is a quantitative trait by measuring colour variation in offspring. I then use a Quantitative Trait Locus (QTL) mapping approach to identify loci controlling the trait in the co-mimics, finding that the genetic basis is not the same in the two species. In H. erato, the colour is strongly sex-linked, while in H. melpomene, we find a large effect locus on chromosome 3, plus a number of putative small effect loci in each species. Therefore, iridescence in Heliconius is not an example of repeated gene reuse. I then show that both iridescent colour and pigment colour are sexually dimorphic in H. -
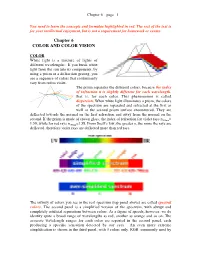
Chapter 6 COLOR and COLOR VISION
Chapter 6 – page 1 You need to learn the concepts and formulae highlighted in red. The rest of the text is for your intellectual enjoyment, but is not a requirement for homework or exams. Chapter 6 COLOR AND COLOR VISION COLOR White light is a mixture of lights of different wavelengths. If you break white light from the sun into its components, by using a prism or a diffraction grating, you see a sequence of colors that continuously vary from red to violet. The prism separates the different colors, because the index of refraction n is slightly different for each wavelength, that is, for each color. This phenomenon is called dispersion. When white light illuminates a prism, the colors of the spectrum are separated and refracted at the first as well as the second prism surface encountered. They are deflected towards the normal on the first refraction and away from the normal on the second. If the prism is made of crown glass, the index of refraction for violet rays n400nm= 1.59, while for red rays n700nm=1.58. From Snell’s law, the greater n, the more the rays are deflected, therefore violet rays are deflected more than red rays. The infinity of colors you see in the real spectrum (top panel above) are called spectral colors. The second panel is a simplified version of the spectrum, with abrupt and completely artificial separations between colors. As a figure of speech, however, we do identify quite a broad range of wavelengths as red, another as orange and so on. -

The Superfamily Calopterygoidea in South China: Taxonomy and Distribution. Progress Report for 2009 Surveys Zhang Haomiao* *PH D
International Dragonfly Fund - Report 26 (2010): 1-36 1 The Superfamily Calopterygoidea in South China: taxonomy and distribution. Progress Report for 2009 surveys Zhang Haomiao* *PH D student at the Department of Entomology, College of Natural Resources and Environment, South China Agricultural University, Guangzhou 510642, China. Email: [email protected] Introduction Three families in the superfamily Calopterygoidea occur in China, viz. the Calo- pterygidae, Chlorocyphidae and Euphaeidae. They include numerous species that are distributed widely across South China, mainly in streams and upland running waters at moderate altitudes. To date, our knowledge of Chinese spe- cies has remained inadequate: the taxonomy of some genera is unresolved and no attempt has been made to map the distribution of the various species and genera. This project is therefore aimed at providing taxonomic (including on larval morphology), biological, and distributional information on the super- family in South China. In 2009, two series of surveys were conducted to Southwest China-Guizhou and Yunnan Provinces. The two provinces are characterized by karst limestone arranged in steep hills and intermontane basins. The climate is warm and the weather is frequently cloudy and rainy all year. This area is usually regarded as one of biodiversity “hotspot” in China (Xu & Wilkes, 2004). Many interesting species are recorded, the checklist and photos of these sur- veys are reported here. And the progress of the research on the superfamily Calopterygoidea is appended. Methods Odonata were recorded by the specimens collected and identified from pho- tographs. The working team includes only four people, the surveys to South- west China were completed by the author and the photographer, Mr. -

Seeing Through Moving Eyes
bioRxiv preprint doi: https://doi.org/10.1101/083691; this version posted June 1, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Seeing through moving eyes - microsaccadic information sampling provides 2 Drosophila hyperacute vision 3 4 Mikko Juusola1,2*‡, An Dau2‡, Zhuoyi Song2‡, Narendra Solanki2, Diana Rien1,2, David Jaciuch2, 5 Sidhartha Dongre2, Florence Blanchard2, Gonzalo G. de Polavieja3, Roger C. Hardie4 and Jouni 6 Takalo2 7 8 1National Key laboratory of Cognitive Neuroscience and Learning, Beijing, Beijing Normal 9 University, Beijing 100875, China 10 2Department of Biomedical Science, University of Sheffield, Sheffield S10 T2N, UK 11 3Champalimaud Neuroscience Programme, Champalimaud Center for the Unknown, Lisbon, 12 Portugal 13 4Department of Physiology Development and Neuroscience, Cambridge University, Cambridge CB2 14 3EG, UK 15 16 *Correspondence to: [email protected] 17 ‡ Equal contribution 18 19 Small fly eyes should not see fine image details. Because flies exhibit saccadic visual behaviors 20 and their compound eyes have relatively few ommatidia (sampling points), their photoreceptors 21 would be expected to generate blurry and coarse retinal images of the world. Here we 22 demonstrate that Drosophila see the world far better than predicted from the classic theories. 23 By using electrophysiological, optical and behavioral assays, we found that R1-R6 24 photoreceptors’ encoding capacity in time is maximized to fast high-contrast bursts, which 25 resemble their light input during saccadic behaviors. Whilst over space, R1-R6s resolve moving 26 objects at saccadic speeds beyond the predicted motion-blur-limit. -

Butterflies from the Middle Eocene: the Earliest Occurrence of Fossil Papilionoidea (Lepidoptera)
THE PEARCE- SELLARDS Sctks NUMBER 29 BUTTERFLIES FROM THE MIDDLE EOCENE: THE EARLIEST OCCURRENCE OF FOSSIL PAPILIONOIDEA (LEPIDOPTERA) Christopher J. Durden and Hugh Rose 1978 Texas Memorial Museum/2400 Trinity/Austin, Texas 78705 W. W. Newcomb, Director The Pearce-Sellards Series is an occasional, miscellaneous series of brief reports of museum and museum associated field investigations and other research. Its title seeks to commemorate the first two directors of the Texas Memorial Museum, now both deceased: J. E. Pearce and Dr. E. H. Sellards, professors of anthropology and geology respectively, of The University of Texas. A complete list of Pearce-Sellards papers, as well as other publica- tions of the museum, will be sent upon request. BUTTERFLIES FROM THE MIDDLE EOCENE: THE EARLIEST OCCURRENCE OF FOSSIL PAPILIONOIDEA (LEPIDOPTERA) 1 Christopher J. Durden 2 and Hugh Rose 3 ABSTRACT Three fossil butterflies recently collected from the Green River Shale of Colorado extend the known range of Rhopalocera eight to ten million years back, to 48 Ma. Praepapilio Colorado n. g., n. sp., and P. gracilis n. sp. are primitive Papilionidae related to the modern Baronia brevicornis Salvin, but they require a new subfamily, Praepapilioninae. Riodinella nympha n. g., n. sp. is a primitive member of the Lycaenidae, related to modern Ancyluris, Riodina, and Rhetus, in the tribe Riodinidi. INTRODUCTION With approximately 194,000 living species, the Lepidoptera is, after the Coleoptera with some 350,000, species, the second most diverse order of organisms. It is underrepresented in the fossil record (Scudder 1875, 1891, 1892; Handlirsch 1925;Mackay 1970;Kuhne 1973; Shields 1976).