Lineage Development in a Patient Without Goblet, Paneth, and Enteroendocrine Cells: a Clue for Intestinal Epithelial Differentiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Study of Mucin Turnover in the Small Intestine by in Vivo Labeling Hannah Schneider, Thaher Pelaseyed, Frida Svensson & Malin E
www.nature.com/scientificreports OPEN Study of mucin turnover in the small intestine by in vivo labeling Hannah Schneider, Thaher Pelaseyed, Frida Svensson & Malin E. V. Johansson Mucins are highly glycosylated proteins which protect the epithelium. In the small intestine, the goblet Received: 23 January 2018 cell-secreted Muc2 mucin constitutes the main component of the loose mucus layer that traps luminal Accepted: 26 March 2018 material. The transmembrane mucin Muc17 forms part of the carbohydrate-rich glycocalyx covering Published: xx xx xxxx intestinal epithelial cells. Our study aimed at investigating the turnover of these mucins in the small intestine by using in vivo labeling of O-glycans with N-azidoacetylgalactosamine. Mice were injected intraperitoneally and sacrifced every hour up to 12 hours and at 24 hours. Samples were fxed with preservation of the mucus layer and stained for Muc2 and Muc17. Turnover of Muc2 was slower in goblet cells of the crypts compared to goblet cells along the villi. Muc17 showed stable expression over time at the plasma membrane on villi tips, in crypts and at crypt openings. In conclusion, we have identifed diferent subtypes of goblet cells based on their rate of mucin biosynthesis and secretion. In order to protect the intestinal epithelium from chemical and bacterial hazards, fast and frequent renewal of the secreted mucus layer in the villi area is combined with massive secretion of stored Muc2 from goblet cells in the upper crypt. Te small intestinal epithelium is constantly aiming to balance efective nutritional uptake with minimal damage due to exposure to ingested, secreted and resident agents. -

Parietal Cell Hyperplasia Induced by Long-Term Administration of Antacids to Rats
Gut: first published as 10.1136/gut.19.9.798 on 1 September 1978. Downloaded from Gut, 1978, 19, 798-801 Parietal cell hyperplasia induced by long-term administration of antacids to rats G. MAZZACCA1, F. CASCIONE, G. BUDILLON, L. D'AGOSTINO, L. CIMINO, AND C. FEMIANO From the Division of Gastroenterology, 2nd School ofMedicine, University ofNaples, and the Department ofPathology, Institute for Tumoral Diseases, Naples, Italv SUMMARY Suspension of magnesium and aluminum hydroxide (30-60 mEq/24 h) or a comparable volume of water was orally administered by gastric intubation to two groups of 20 male wistar rats each over 60 days. The antacid treatment led to a significant increase in the height (0-464 + 0-02 mm v. 0-318 ± 0 06) and in the volume (472 ± 32 mm3 v. 328 ± 45) of the fundic mucosa ofthe stomach, in the average count of parietal cells per unit area of the mucosa (32-37 ± 1 8 v. 22-3 + 1 6), and in the total parietal cell population of the stomach (53-6 + 3.5 x 106 v. 43-2 + 3.7 x 106). Furthermore fasting serum gastrin concentration was significantly higher in the antacid treated rats (81.2 + 7.4 pg/ml) than in control animals (56-9 ± 6-9 pg/ml). It is known that a feedback mechanism governs the The animals of group B received each time and by relationship between antral gastrin release and the same procedure a comparable volume of water. intraluminal gastric pH (Walsh et al., 1975). During the night each animal was housed in a Furthermore, gastrin exerts a trophic influence on separate cage and the antacid suspension was the the gastric mucosa with increase in the parietal cell only drinking fluid available to the rats of group A. -

Overview of Gastrointestinal Function
Overview of Gastrointestinal Function George N. DeMartino, Ph.D. Department of Physiology University of Texas Southwestern Medical Center Dallas, TX 75390 The gastrointestinal system Functions of the gastrointestinal system • Digestion • Absorption • Secretion • Motility • Immune surveillance and tolerance GI-OP-13 Histology of the GI tract Blood or Lumenal Serosal Side or Mucosal Side Structure of a villus Villus Lamina propria Movement of substances across the epithelial layer Tight junctions X Lumen Blood Apical membrane Basolateral membrane X X transcellular X X paracellular GI-OP-19 Histology of the GI tract Blood or Lumenal Serosal Side or Mucosal Side Motility in the gastrointestinal system Propulsion net movement by peristalsis Mixing for digestion and absorption Separation sphincters Storage decreased pressure GI-OP-42 Intercellular signaling in the gastrointestinal system • Neural • Hormonal • Paracrine GI-OP-10 Neural control of the GI system • Extrinsic nervous system autonomic central nervous system • Intrinsic (enteric) nervous system entirely with the GI system GI-OP-14 The extrinsic nervous system The intrinsic nervous system forms complete functional circuits Sensory neurons Interneurons Motor neurons (excitatory and inhibitory) Parasympathetic nerves regulate functions of the intrinsic nervous system Y Reflex control of gastrointestinal functions Vago-vagal Afferent reflex Salivary Glands Composition of Saliva O Proteins α−amylase lactoferrin lipase RNase lysozyme et al mucus O Electrolyte solution water Na+ , K + - HCO3 -

Paneth Cell Α-Defensins HD-5 and HD-6 Display Differential Degradation Into Active Antimicrobial Fragments
Paneth cell α-defensins HD-5 and HD-6 display differential degradation into active antimicrobial fragments D. Ehmanna, J. Wendlera, L. Koeningera, I. S. Larsenb,c, T. Klaga, J. Bergerd, A. Maretteb,c, M. Schallere, E. F. Stangea, N. P. Maleka, B. A. H. Jensenb,c,f, and J. Wehkampa,1 aInternal Medicine I, University Hospital Tübingen, 72076 Tübingen, Germany; bQuebec Heart and Lung Institute, Department of Medicine, Faculty of Medicine, Cardiology Axis, Laval University, G1V 4G5 Quebec, QC, Canada; cInstitute of Nutrition and Functional Foods, Laval University, G1V 4G5 Quebec, QC, Canada; dElectron Microscopy Unit, Max-Planck-Institute for Developmental Biology, 72076 Tübingen, Germany; eDepartment of Dermatology, University Hospital Tübingen, 72076 Tübingen, Germany; and fNovo Nordisk Foundation Center for Basic Metabolic Research, Section for Metabolic Genomics, Faculty of Health and Medical Sciences, University of Copenhagen, 1165 Copenhagen, Denmark Edited by Lora V. Hooper, The University of Texas Southwestern, Dallas, TX, and approved January 4, 2019 (received for review October 9, 2018) Antimicrobial peptides, in particular α-defensins expressed by Paneth variety of AMPs but most abundantly α-defensin 5 (HD-5) and -6 cells, control microbiota composition and play a key role in intestinal (HD-6) (2, 17, 18). These secretory Paneth cells are located at the barrier function and homeostasis. Dynamic conditions in the local bottom of the crypts of Lieberkühn and are highly controlled by microenvironment, such as pH and redox potential, significantly af- Wnt signaling due to their differentiation and their defensin regu- fect the antimicrobial spectrum. In contrast to oxidized peptides, lation and expression (19–21). -

New Developments in Goblet Cell Mucus Secretion and Function
REVIEW nature publishing group New developments in goblet cell mucus secretion and function GMH Birchenough1, MEV Johansson1, JK Gustafsson1, JH Bergstro¨m1 and GC Hansson1 Goblet cells and their main secretory product, mucus, have long been poorly appreciated; however, recent discoveries have changed this and placed these cells at the center stage of our understanding of mucosal biology and the immunology of the intestinal tract. The mucus system differs substantially between the small and large intestine, although it is built around MUC2 mucin polymers in both cases. Furthermore, that goblet cells and the regulation of their secretion also differ between these two parts of the intestine is of fundamental importance for a better understanding of mucosal immunology. There are several types of goblet cell that can be delineated based on their location and function. The surface colonic goblet cells secrete continuously to maintain the inner mucus layer, whereas goblet cells of the colonic and small intestinal crypts secrete upon stimulation, for example, after endocytosis or in response to acetyl choline. However, despite much progress in recent years, our understanding of goblet cell function and regulation is still in its infancy. THE INTESTINE system of mucus covering the epithelium. There is a The gastrointestinal tract is a remarkable organ. Not only can it two-layered mucus system in the stomach and colon and a digest most of our food into small components, but it is also single-layered mucus in the small intestine.5 The mucus layers filled with kilograms of microbes that live in stable equilibrium in these three regions perform their protective function using with us and our immune system. -

The Transcriptional Repressor Blimp1/Prdm1 Regulates Postnatal Reprogramming of Intestinal Enterocytes
The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes James Harpera, Arne Moulda, Robert M. Andrewsb, Elizabeth K. Bikoffa, and Elizabeth J. Robertsona,1 aSir William Dunn School of Pathology, University of Oxford, Oxford OX1 3RE, United Kingdom; and bWellcome Trust Sanger Institute, Genome Campus, Hinxton-Cambridge CB10 1SA, United Kingdom Edited by Brigid L. M. Hogan, Duke University Medical Center, Durham, NC, and approved May 19, 2011 (received for review April 13, 2011) Female mammals produce milk to feed their newborn offspring rise to the developing crypts. The crypt derived adult enterocytes before teeth develop and permit the consumption of solid food. that emerge at postnatal day (P) 12 and repopulate the entire Intestinal enterocytes dramatically alter their biochemical signature epithelium lack Blimp1 expression. Conditional inactivation of during the suckling-to-weaning transition. The transcriptional re- Blimp1 results in globally misregulated gene expression patterns, pressor Blimp1 is strongly expressed in immature enterocytes in and compromises small intestine (SI) tissue architecture, nutrient utero, but these are gradually replaced by Blimp1− crypt-derived uptake, and survival of the neonate. In combination with tran- adult enterocytes. Here we used a conditional inactivation strategy scriptional profiling, the present experiments demonstrate that to eliminate Blimp1 function in the developing intestinal epithe- Blimp1/Prdm1 orchestrates orderly and extensive reprogramming lium. There was no noticeable effect on gross morphology or of the postnatal intestinal epithelium. formation of mature cell types before birth. However, survival of mutant neonates was severely compromised. Transcriptional Results profiling experiments reveal global changes in gene expression Down-Regulated Blimp1 Expression in Crypt Progenitors Beginning at patterns. -

Expression of Cell Membrane Complement Regulatory Glycoproteins Along the Normal and Diseased Human Gastrointestinal Tract
522 Gut 1998;42:522–529 Expression of cell membrane complement regulatory glycoproteins along the normal and diseased human gastrointestinal tract Gut: first published as 10.1136/gut.42.4.522 on 1 April 1998. Downloaded from A E Berstad, P Brandtzaeg Abstract tract. Indeed, activated complement has been Background/Aims—Uncontrolled comple- detected in lesions of inflammatory bowel ment activation may be of immunopatho- disease,2–4 coeliac disease,5 and recently also in logical importance in inflammatory Helicobacter pylori gastritis.6 diseases of the gastrointestinal tract. Ex- Certain complement activation products, pression of membrane bound factors that notably the C3b fragment and the C5b-7 com- regulate complement activation was there- plex, can on their own—that is, without associ- fore studied in situ. ated antibody—bind to any nearby cell Methods—Frozen tissue specimens were membrane.7 Therefore it is crucial that com- obtained from patients with Helicobacter plement activity is tightly regulated. Mam- pylori gastritis, coeliac disease, Crohn’s malian cells are protected from complement disease, or ulcerative colitis, and from induced damage by a family of cell membrane histologically normal controls. Sections complement regulatory glycoproteins that were examined by immunofluorescence downregulate activation of homologous com- with monoclonal antibodies to protectin plement on their cell surface.8 Protectin (CD59), decay accelerating factor (DAF), (CD59) is broadly distributed on cells of and membrane cofactor protein (MCP). haemopoietic and non-haemopoietic origin79; Results—Protectin and MCP were widely it inhibits the formation of terminal comple- expressed in normal and diseased mu- ment complex by preventing the binding of C9 cosae. -

Enterocyte Proliferation and Intracellular Bacteria in Animals
Gut 1994; 35: 1483-1486 1483 PROGRESS REPORT Gut: first published as 10.1136/gut.35.10.1483 on 1 October 1994. Downloaded from Enterocyte proliferation and intracellular bacteria in animals S McOrist, C J Gebhart, G H K Lawson Considerable proliferation of enterocytes, candidates for the bacteria involved.2 Recent forming adenomatous growths within the work shows that the intracellular bacteria are a intestinal mucosa, is a consistent feature of a new genus of obligate intracellular bacteria, number of enteric conditions in animals, now living within enterocytes. This report describes referred to under the general heading of pro- recent advances in the taxonomic status of the liferative enteropathy. These were originally bacteria and its association with disease patho- described in pigs as adenomas ofthe ileum and genesis in animals, focusing on how this can colon1 and this condition was found to have a aid an understanding of enterocyte prolifera- worldwide occurrence.2 Similar conditions tion. were subsequently described in a number of other animal species, again under the general heading of proliferative enteropathy or Intracellular bacteria enteritis. The degree, however, of mucosal The intracellular bacteria in proliferative proliferation, corresponding to a description of enteropathy in animals have been closely hyperplasia, through adenoma to a more studied in the pig and hamster species, in carcinomatous form, varies between species. which the disease is common and widespread. Occasional reports of the condition in the fox,3 Clinical diagnosis of infection and disease is, horse,4 and guinea pig5 describe a hyperplastic however, difficult2 and the disease might be condition of the mucosa whereas hamsters6 7 recognised more widely if diagnostic tools develop adenomatous lesions similar to the became available. -

Regulatory Network Analysis of Paneth Cell and Goblet Cell Enriched Gut Organoids Using Transcriptomics Approaches
bioRxiv preprint doi: https://doi.org/10.1101/575845; this version posted August 16, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Regulatory network analysis of Paneth cell and goblet cell enriched gut organoids using transcriptomics approaches Treveil A1,2*, Sudhakar P1,3*, Matthews Z J4 *, Wrzesinski T1, Jones E J1,2, Brooks J1,2,4,5, Olbei M1,2, Hautefort I1, Hall L J2, Carding S R2,4, Mayer U6, Powell P P4, Wileman T2,4, Di Palma F1, Haerty W1,#, Korcsmáros T1,2# 1 Earlham Institute, Norwich Research Park, Norwich, Norfolk, NR4 7UZ, UK 2 Quadram Institute, Norwich Research Park, Norwich, Norfolk, NR4 7UA, UK 3 Department of Chronic Diseases, Metabolism and Ageing, KU Leuven, Belgium 4 Norwich Medical School, University of East Anglia, Norwich, Norfolk, NR4 7TJ, UK 5 Department of Gastroenterology, Norfolk and Norwich University Hospitals, Norwich, UK 6 School of Biological Sciences, University of East Anglia, Norwich, Norfolk, NR4 7TJ, UK * Equal contributions # Joint corresponding authors Corresponding author contact details: Dr Tamas Korcsmaros ORCID id : https://orcid.org/0000-0003-1717-996X [email protected] Phone : 0044-1603450961 Fax : 0044-1603450021 Address: Earlham Institute, Norwich Research Park, Norwich, NR4 7UZ, UK 1 bioRxiv preprint doi: https://doi.org/10.1101/575845; this version posted August 16, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Effect of Chronic Ethanol Ingestion on Enterocyte Turnover in Rat Small Intestine
Gut: first published as 10.1136/gut.28.1.52 on 1 January 1987. Downloaded from Gut, 1987, 28, 52-55 Effect of chronic ethanol ingestion on enterocyte turnover in rat small intestine R MAZZANTI AND W J JENKINS From the Department of' Medicine, Royal Free Hospital School of Medicine, Rowland Hill Street, Londlon SUMMARY Whether chronic ethanol ingestion significantly damages the small intestine remains controversial. To clarify this we have analysed the morphology of the small intestinal epithelium and quantified its renewal in chronically ethanol fed rats. Twenty adult male rats were pair fed for 28 days a nutritionally adequate liquid diet containing either ethanol as 36% of total calories or an isocaloric diet in which fat substituted for ethanol. Crypt cell production rate was determined in the jejunum and ileum by the metaphase arrest method. Weight gain and small intestinal morphology were similar in ethanol fed and control rats, but enterocyte turnover was significantly reduced in the jejunum (p<O.05) and ileum (p<0.0l) of the ethanol fed rats. This effect of ethanol on the small intestine is probably systemic rather than local, because the changes in jejunum and ileum were similar, and it may contribute to the development of malnutrition in chronic alcoholics. A variety of changes in intestinal function have Methods been reported after acute or habitual alcohol consumption.' Impaired absorption of vitamins,25 ANIMALS sugars6 7 and amino acids8 9 has been shown in Twenty adult male Sprague-Dawley rats, which http://gut.bmj.com/ the presence of ethanol, and the chronic consump- initially weighed between 210 g and 310 g, were tion of ethanol is often associated with malnutrition maintained in single cages on a 12 hour light-dark and diarrhoea, but whether these are caused by cycle at 21 ±1°C room temperature. -
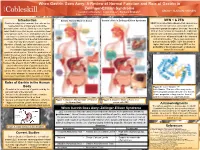
A Review of Normal Function and Role of Gastrin in Zollinger-Ellison
When Gastrin Goes Awry: A Review of Normal Function and Role of Gastrin in Zollinger-Ellison Syndrome Leigha LaTourette1, Janel Cross2, Barbara Brabetz2 Department of Plant and Animal Science1, Department of Natural Sciences and Mathematics2 Introduction Gastrin: Normal Mode of Action Gastrin’s Role in Zollinger-Ellison Syndrome MEN 1 & ZES Gastrin is a digestive hormone that acts on the MEN1 is an inheritable disease that causes over neuroendocrine and parietal cells of the secretion of hormones via tumors on the gastrointestinal tract to ultimately secrete gastric endocrine glands throughout the body.4 Around a acid. Gastrin secretion begins even before food 30% of these tumors are found to be malignant. is consumed, as the mere anticipation of a meal ZES is commonly associated with the MEN1 due can lead to a series of events ending in the to the presence of tumors found in the stomach creation of the gastric acid needed to breakdown and small intestine.4 These gastrointestinal foods. Not only does Gastrin support the tumors caused by MEN 1 give the person a digestion of foods, but it also stimulates growth, higher likelihood of contracting ZES, as the secretion, blood flow, and acts as a defense probability of having pancreatic or duodenal mechanism against bacteria in the tumors is increased.4 gastrointestinal system. When the production of Gastrin becomes overt, major consequences like that of Zollinger Ellison Syndrome (ZES). ZES is an extremely rare disease occurring in people between the ages of 30-60.4 ZES is related to the uncontrolled secretion of gastrin due to the presence of certain pancreatic or duodenal tumors. -

The Digestive and Endocrine Systems Examination
The Digestive and Lesson 3 Endocrine Systems Lesson 3 ASSIGNMENT 6 Read in your textbook, Clinical Anatomy and Physiology for Veterinary Technicians, pages 358–377, 436, and 474–475. Then read Assignment 6 in this study guide. Introduction The endocrine system involves the secretion of chemicals called hormones by glands within the body. These chemicals control bodily functions, often at locations very distant from the gland that secreted the chemical. The opposite of endocrine is exocrine, which involves the secretion of sub- stances into spaces outside the body. The glands in the skin and gastrointestinal tract are examples of exocrine organs. (The lumen of the intestine is a space that technically isn’t “within” the body, because it’s continuous with the outside environment via the mouth and anus.) The pancreas is unique in being both an endocrine gland and an exocrine gland. The endocrine function is the secretion of substances such as insulin, which metabolizes sugar. The exocrine function involves the secretion of digestive enzymes into the duodenum. Many endocrine organs exist throughout the body (see Table 15-2 on page 360 of your textbook). While they’re all classified as endocrine glands, their anatomy and functions aren’t necessarily similar or even remotely related. Therefore, there isn’t really a grand organizational scheme to this sys- tem as there is with some of the other body systems. The glands are considered together only because their means of secretion is similar. All endocrine glands secrete hormones in the form of proteins that travel via the blood to the target organ for that particular hormone.