Pictorial Representations of Cordite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Recreation and Ballistic Evaluation of Otto Schneeloch's Firearm Curiosity
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 3-5-2014 A Recreation and Ballistic Evaluation of Otto Schneeloch's Firearm Curiosity - The .307 Triangular Amber Nicole Shukitis University of South Florida, [email protected] Follow this and additional works at: https://scholarcommons.usf.edu/etd Part of the Mechanical Engineering Commons Scholar Commons Citation Shukitis, Amber Nicole, "A Recreation and Ballistic Evaluation of Otto Schneeloch's Firearm Curiosity - The .307 rT iangular" (2014). Graduate Theses and Dissertations. https://scholarcommons.usf.edu/etd/5125 This Thesis is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. A Recreation and Ballistic Evaluation of Otto Schneeloch’s Firearm Curiosity – The .307 Triangular by Amber Shukitis A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Mechanical Engineering Department of Mechanical Engineering College of Engineering University of South Florida Major Professor: Stuart Wilkinson, Ph.D. Nathan Gallant, Ph.D. Rasim Guldiken, Ph.D. Date of Approval: March 5, 2014 Keywords: Triangular Cross Sectioned Bullets, Uniquely Shaped Projectiles, Twisted Triangular Bore, Triangular Direct Metal Laser Sintering Barrel, Triangular Bored Revolver Copyright © 2014, Amber Shukitis TABLE -

Attachment L
Attachment L - Ammunition Specification Details The ammunition specifications below correspond to the line items, by sub-category in the Ammunition Category in Attachment C – Cost Worksheet. Sub-Category – Speer Line Item #1 Lawman Ammo #53652 Line Item #2 Gold Dot Ammo #53617 Caliber: .40 S&W Caliber: 9mm Bullet Type: Total Metal Jacket (TMJ) Bullet Type: +P Hollow Point Weight: 180 grain Weight: 124 grain Casing: Brass Casing: Nickel Plated Brass Rounds Per Case: 1000 Rounds Per Case: 1000 Line Item #3 Lawman Ammo #53620 Line Item #4 Lawman Ammo #53955 Caliber: 9mm Luger Caliber: .40 S&W Bullet Type: Total Metal Jacket (TMJ) Bullet Type: Total Metal Jacket (TMJ) Weight: 147 grain Weight: 165 grain Casing: Brass Casing: Brass Rounds Per Case: 1000 Rounds Per Case: 1000 Line Item #5 Lawman Ammo #53650 Line Item #6 Gold Dot Ammo #53962 Caliber: 9mm Luger Caliber: .40 S&W Bullet Type: Total Metal Jacket (TMJ) Bullet Type: Hollow Point Weight: 115 grain Weight: 180 grain Casing: Brass Casing: Nickel Plated Brass Rounds Per Case: 1000 Rounds Per Case: 1000 Line Item #7 Lawman Ammo #53651 Line Item #8 Lawman Ammo #53653 Caliber: 9mm Luger Caliber: 45 ACP Auto Bullet Type: Total Metal Jacket (TMJ) Bullet Type: Total Metal Jacket (TMJ) Weight: 124 grain Weight: 230 grain Casing: Brass Casing: Brass Rounds Per Case: 1000 Rounds Per Case: 1000 Line Item #9 Gold Dot Ammo #53619 Line Item #10 Gold Dot Ammo #53970 Caliber: 9mm Luger Caliber: .40 S&W Bullet Type: Jacketed Hollow Point Bullet Type: Jacketed Hollow Point Weight: 147 grain Weight: 165 -

HOW to LOAD COWBOY FAST DRAW CARTRIDGES By: Cal Eilrich A.K.A
HOW TO LOAD COWBOY FAST DRAW CARTRIDGES By: Cal Eilrich a.k.a. Quick Cal, Executive Director CFDA Revised: November 27th, 2016 In 2007, we began researching various methods used to load Cowboy Fast Draw Cartridges for CFDA Titled Championship events. Everyone seemed to have their own formula. Some loaded them by hand and some loaded them on progressive loading presses. We found a wide variety of results, some even within the same tournament in which there was found 300+ (fps) variations in velocities. This meant that championships could have been decided because of ammunition variations of as much as 2 hundredths of a second (.020), which is a huge amount of time in Cowboy Fast Draw. We simply had to do better. In reality there will always be some variations in velocities even in factory live ammunition, at 15 – 20 fps. Bullseye shooters use techniques such as matched brass, matched bullets and a trickle powder charge to get it tighter than that. We also have to keep in mind that wax bullets, due to density variances, can never be manufactured and sized as exact as lead or jacketed bullets can be. After extensive testing, we have found that it is possible to reduce the velocity variances to around 30 – 40 fps, which only amounts to a few thousandths of a second. This is true with both CFD Cartridges and Shotgun Primer Loads. This article is about addressing, not only various components, but also loading techniques, and the best equipment used to load them. • Brass • Brass is the first step in producing consistent cartridges, make sure that you tumble them thoroughly, before removing the primers. -

Dictionary of Explosives
DICTIONARY OF EXPLOSIVES BY ARTHUR MARSHALL A .C .G j., F.I.C., F.C.S. CHEMICAL INSPECTOR INDIAN ORDNANCE DEPARTMENT PHILADELPHIA P. BLAKISTON’S SON & CO. 1012 WALNUT STREET 1920 Printed in Great Britain INTRODUCTION It is a generation since a dictionary of explosives has been published, and, in the meantime, many new explosives have been introduced. It is hoped, therefore, that this small volume, giving concise information about these special materials, may prove useful to those who have to deal with them. In Cundill and Thomson’s “ Dictionary of Explosives,” issued in 1895, there arc many entries of the names of inventors and of mixtures which had been proposed but have never been used commercially, nor are likely to be. As modem explosives were then in their infancy, it was no doubt wise to insert all the available information whether it appeared to be important or not; but now it seems to me better to restrict the scope of the dictionary so as to keep its size within moderate limits. Practically only explosives with special or proprietary names are therefore dealt with here. For information concerning chemical substances, such as the nitro-toluenes and other nitro-compounds, reference should be made to the text-books on explosives and chemistry. A few words may, however, be said here about the nitro- celluloses. These are made by treating cellulose with a mix ture of nitric and sulphuric acids, and then purifying the product by washing it thoroughly with hot water. The variety of cellulose most used for this purpose is cotton, and the product obtained from it is frequently called nitrocotton, three special varieties of which are collodion cotton, pyro- collodion and guncotton (q. -

The Gold Standard a Week, All Year Long
Speer customers will now see a more aggressive logo on its new packaging to signify the company’s forward progression. to create better products year after year. Speer’s product lineup centers on rifle and Speer and its sister company handgun ammunition for law enforcement and CCI currently operate out of self-defense, as well as handloading components. three facilities encompassing 400 acres in Lewiston, with more than 350,000 square feet of manufacturing space oper- ated by 1,100 employees. They run 24 hours a day, seven days The Gold Standard a week, all year long. Speer’s relentless dedication to quality and innovation pays big dividends for its customers THE CHOICE OF PROFESSIONALS any large industrial businesses began as smaller undertakings, driven by a for- “There’s a big reason Speer ward-thinking innovator who saw opportunity where others did not. Such is the is the number one choice of case with Speer, founded by Vernon Speer during World War II when reloaders were law enforcement professionals not able to get the components they needed from ammo companies committed to across the country,” said Jason supplying the U.S. war effort. Vanderbrink, president of M Speer. “We service more than 80 percent of law enforcement nationwide. We also have many international contracts. Why? Because we build ammo that is extremely dependable; because we focus on terminal ballistics; and because we are the masters of meeting the A born tinkerer, Speer Idaho, by an experienced and nose, the Grand Slam bullet FBI protocol testing, which devised a way to take spent dedicated work force, but the is engineered to tear through is the gold standard of bullet .22 brass cases and form them original product lines have the thick hides, heavy bones terminal performance.” into bullet jackets. -

Load Guide for Rifle and Handgun
EDITION 3.5 EDITION 30 AR, 30 T/C, and 6mmx45 and T/C, 30 AR, 30 New data for: for: data New • The most up-to-date and comprehensive 223 Rem data available data Rem 223 comprehensive and up-to-date most The • INCLUDES: GUN H D N A AND RIFLE FOR GUIDE LOAD PO Box 158 • Miles City, Montana 59301 Other superior products available ONLY from Western Powders include: RELOADING POWDERS Gun Care Products EDITION 3.5 WARNINGS COMPONENT WARNINGS This guide is intended to be used as a reference. Each individual Primers handloader must determine what is the best and safest load for their 1. NEVER MIX PRIMER BRANDS from different manufacturers; equipment. The loads described in this guide were generated at the 2. Store primers in their original packaging(s) in a cool, dry place. ballistics test facilities of Western Powders, Inc. in accordance with Exposure to heat causes primer deterioration; SAAMI (Shooting Arms and Ammunition Institute) guidelines. All 3. Do not stockpile primers or store in bulk. Storing primers in this loads are fired through test barrels and individual results fired through manner can lead to mass detonation if a primer ignites; different firearms may vary. The handloader is cautioned to read and 4. Do not de-cap live or new primers - fire them in the appropriate follow safe reloading practices such as those outlined in the NRA gun and then de-cap; Guide to Reloading before attempting to reload any cartridge. 5. For best results, use the mildest primer consistent with good ignition; 6. -

GUNS Magazine July 1958
JULY 1958 50c FINEST IN THE FIREARMS FIELD SHOOTING THE NEW ARMY RIFLE HUNTING SunnTING ADVENTURE 1 Gun* 0 Ammo Gun*  Ammo I AMERICA'S GREATEST SHOOTER'S BARGAINS 1 ORIGINAL U. S. MI903 SPRINGFIELDS These are very rare and ultimately desirable ZE GUN OF ZE MONTH original low numbered receivers produced by L'. S. Govt. during WWII with 03A3 latest type com- ponents but with original precision sights and m- low numbered receivers. An official version that's an absolute "Must" for all Springfield men. U. S. Ye Old Is all heart! Hunter unfired condition throughout! ORDER NC'W AN" WARNING! American shooters! You may .s!tbIect CAL. .30-06 ONLY $29.95 SAVE! $;;gfi,$;;u;.g,ygiym~ ~~A$;;[c;~~;&~$ - . Near NEW Condition ! ! ! ! ! ! ! ! ! Ye Old Hunter tllustrates all wea5ons bv actual univtouched photographs so you can see how they REAL1 MAGNIFICEPT cwengeu UACTBRPIECEMAUSER RARE-- BRITISH- ENFIELDSÑCAL .303 CAL. 6.5MM Swedish Mauser IT -- ONLY $22.50 SERVICE RIFLE ONLY $14.95 Without an doubt the finest Mauser ever built are these never-hefore- available Swedish magnificent masterpiece long Mausers! Superbly 6.5MM SWEDISH MAUSER. .S5.50 IX~;~~~~~~!pT&?G;egy 6i,5~~v~;~vytcpci;e~; standards unsurpassed anywhere on the earth. A real sweedie by any standards at a GIVEAWAY bargain AUSTRIAN MANNLICHER POLICE CARBINES! !mm6in;6nwiy huliet. superb bras- cases assure proper components for years to come! U. S. KRAGS! U. S. KRAGS! ONLY $16.95 UP!! 1. Original US. Krag Short Rifle (Type '5") 7MM MAUSER (WIN. CTG. C0.1 .S2.50 Gorffeous ori ins1 Winchester 7mm commercial export, ammo In 28 rd. -

Where Real Cowboys Go! D Walking on the Streets of Vegas Are Bow-Legged Then You by Palaver Pete, SASS Life/Regulator #4375 Know They’Re Part of the Rodeo
E N SASS UNIVERSITY ~ Shooting School April 12th @ Buffalo Stampede www.sassnet.com/buffalo S Cowboy ChroniicllDe i NNSNoeoovpvveteeemmmmbbbbeeeerrrr 22 2200000001111 0 CCoowwCbbooywy CbCfohhyrr ooCn(nhiiiicrcllollee n icle gPPPaaagggeee 111 o S o n r e f m e T U o p R p r a A F October 2010 Cowboy Chronicle Pe ageg 1 I o i e L r n s T f 4 o ~ o 9 r d m - a 5 y a 2 ! The Cowboy Chronicle t ) io n The Monthly Journal of the Single Action Sh ooting Society ® Vol. 24 No. 3 © Single Action Shooting Society, Inc. March 2011 The SASS ConvenTion ecember in Las Vegas means Cowboy Heaven. If the Cowboys you see Where Real Cowboys Go! D walking on the streets of Vegas are bow-legged then you By Palaver Pete, SASS Life/Regulator #4375 know they’re part of the Rodeo. If Photos by Black Jack McGinnis, SASS #2041 they’re wearing Rhinestone cov - ered clothing, then you know they The Convention is important See HIGHLIGHTS pages 33-35 are attending and buying at Cow - for many reasons … the TGs boy Christmas. But if they look readdress the rules each year, like real Cowboys and Cowgirls, the Wooly Awards recognize then you know they are attending Wax Bullet Championship recent achievements, the seminars the 9 th Annual SASS Convention. Winners provide new insight, the Wax Bullet Championships recognizes Oh yes, all three events are worth 49’er Hank Hills, WA shooting excellence, and the seeing, but only one permits you to SASS #78028 Hall of Fame recognizes long-time, shoot indoors, and only one fea - B-Western Long Jim OR lasting contributions to the sport tures a Dine-in Ball where the par - Hancock, of Cowboy Action Shooting™. -

Study Unit 6 - Part 1 Stu Dy
STUDY UNIT 6 - PART 1 STU DY DEVELOPMENT AND DESIGN OF THE MODERN RIFLE BULLET UNIT 6 BEGINNING BALLISTICS PA RT The first projectile was likely a rock "minie ball" that the concept of conical bul which some Alley-Oop type, in the dawn of lets really arrived (se~Figure 2). 1 pre-history, bounced off the bean of a baby The perfection of the breechloading sys brontosaurus. \Ne don't know how the belt in tem, and the self-contained metallic cartridge the belfrey impressed the brontosaurus, but which soon' followed, led to the widespread "Alley" may have learned something about adoption of the new "bullet." There was no sectional density. Sooner or later he appreci denying the vast improvement in accuracy ated the fact that the heavier the rock for its and trajectory that the new configuration size, the farther it could be hurled by his hair provided. Also, a long-bodied projectile was ~ matted muscles. necessary for proper seating in the new metal W lic cases (see Figure 3). ...J The concept of hitting a target more or ...J less impersonally and from a distance was es The first bullets were cast of lead. Not ::> tablished, and with it the need for more effic an awful lot was known about ballistics at the CO w ient, longer-ranging projectiles. Through the time, and bullet caliber, weight, and shape ...J ages, in man's never ending quest for a better were governed more by expediency than sci u. way to lay low his enemies and keep his larder entific principle. -
Crooked Creek Order Form SPRING 2016.Xlsx
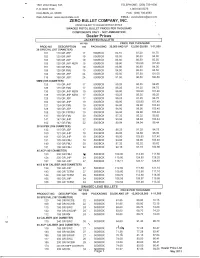
1601 22nd Street, S.E. TELEPHONE: (256)739-1606 P.O. BOX 1188 1-800-545-9376 CULLMAN, AL 35056 FAX: (256)739-4683 Web Address: www.zerobullets.com EMAIL: [email protected] ZERO BULLET COMPANY, INC. PRICES SUBJECT TO CHANGE WITHOUT NOTICE SWAGED PISTOL BULLET PRICES PER THOUSAND COMPONENTS ONLY - NOT AMMUNITION Dealer Prices JACKETED BULLETS WTPER PRICE PER THOUSAND PROD NO DESCRIPTION 1000 PACKAGING 30,000 AND UP 12,000-29,000 1-11,000 38 SPECIAL (357 DIAMETER) 101 110GRJHP 17 500/BOX 82.10 87.20 91.70 102 125GRJHP 19 500/BOX 85.50 90.50 94.00 103 125 GR JSP 19 500/BOX 83.50 88.50 92.50 105 125GRJHPNEW 19 500/BOX 98.90 103.90 107.40 157 130GRFMJ 19 500/BOX 85.90 90.90 94.00 159 125GRFMJ 19 500/BOX 84.50 89.50 93.50 104 158GRJHP 24 500/BOX 92.50 97.50 101.00 119 158 GR JSP 24 500/BOX 91.50 96.50 100.50 9MM (355 DIAMETER) 125 115GRJHP 17 500/BOX 90.25 95.25 99.25 126 115GRFMJ 17 500/BOX 86.25 91.25 94.75 136 125GRJHPNEW 19 500/BOX 98.90 103.90 107.40 135 115GRJHPNEW 17 500/BOX 90.25 95.25 99.25 129 115 GR JSP 17 500/BOX 86.25 91.25 94.75 162 125GRJHP 19 500/BOX 98.90 103.90 107.40 127 124GRFMJ 19 500/BOX 94.90 99.90 103.40 128 125 GR JSP 19 500/BOX 94.90 99.90 103.40 158 125GRFPFMJ 19 500/BOX 94.90 99.90 103.40 137 135GRFMJ 20 500/BOX 87.32 92.32 95.82 147 147GRJHP 22 500/BOX 93.94 98.94 102.44 152 147GRFMJ 22 500/BOX 89.94 94.94 98.44 38 SUPER (356 DIAMETER) 132 115 GR JSP 17 500/BOX 86.25 91.25 94.75 160 121GRJHP 18 500/BOX 89.80 94.80 98.30 161 125GRJHP 19 500/BOX 98.90 103.90 107.40 ' 131 125GRFMJ 19 500/BOX 94.90 99.90 103.40 140 -

The Ricochet
EXECUTIVE OFFICERS President: Terry Abbott Vice President: Jim Neff Secretary: Dave Howe Treasurer: Ed Roberts Executive Officer : Paul Sherman Chief Instructor: Edgar Artiaga ******************** Range Manager: Bill Lagusis Administrative Assistants: Cheryl Mauler Linda Kempton Financial Manager: Marge Abbott Historians: Bob Shell & Jim Decker The Spring 2013 ******************** Rio Salado Sportsman’s Club, Inc. Ricochet 3960 N. Usery Pass Road Mesa, Arizona 85207-9702 Phone: 480-984-3724 480-984-9610 Fax: 480-986-1592 E-MAIL [email protected] WEB www.riosaladosportsmans.com Pete Carstensen, Webmaster A Call for ******************** Assistance! The range is closed on New Years Day, Easter Sunday, Thanksgiv- We need your help. ing Day, and Christmas Day each year. APRIL, MAY, JUNE President that is to work with RSSC to locate and set aside a small piece of property that can be developed into a shooting range. As any of you that are regular visi- Our Executive Officer, Paul Sher- tors to the range have seen, we are wall to man, and myself have already put togeth- wall with shooters on most weekends. er a proposal for a new facility. This facil- This is particularly true on the public ity will be in addition to our existing fa- range and the shotgun range. We continue cility. That proposal was presented at a to see record numbers of first time shoot- meeting between RSSC, Arizona Game ers and our membership continues to and Fish and the Mesa District Ranger for grow. For some time now the BOD has the Tonto. We are not asking for any fi- been exploring options to obtain more nancial assistance, just a location. -

American Handgunner May/June 1980
....,Remington is.a'trademar1< registered in the United States Patent & Tradem<M High Velocity are trademar1<s{lf Remington Arms Company. Inc.• Bridgeport. Conrt. MAY/JUNE,1980 Vol. 5 No. 3-22 FEATURES STAFF THE DEFENSIVE DUO, James D. Mason ..•................. 28 GEORGE E. VON ROSEN The COP And The TP-70 ... Two Stainless Publisher Packages of Power . .. One Small, One Big Caliber . JEROME RAKUSAN HANDGUN HUNTING-AFRICAN STYLE, AI Venter 32 Editorial Director Ever Wonder What It Would Be Like Shooting A .44 Maggie Deep In The Dark Continent? MIKE THILL YOUR ACCURACY JOB, John Lawson , 38 Managing Editor This Author Turned Gunsmith Tells It Like It KEVIN E. STEELE Is To Make Sure You Really Get An Accuracy Job. Associate Editor COACH A WOMEN'S PISTOL TEAM, A. C. Greenstein . 40 Author Lets It All Hang Out In This Personal SYDNEY BARKER Confession Of A Man Telling Today's Woman What To Do. Art Director THE 19791HMSA INTERNATIONAL CHAMPIONSHIPS, FERNANDO M. MARTINEZ Philip C. Briggs .............................. .. 44 Assistant Art Director Who Took Away All The Marbles At The Steel Turkey JOAN HUBERT Shootout And Just How It All Happened. MAGGIE LOOS SLING, SLANG, SLUNG, Philip C. Briggs 47 Art Assistants By Now You Might Have Found A Holster For That Silhouette Gun . .. But What About Using A Sling? STEVE LIPSKY 547 YARD RAMS, J. D. Jones . 66 Advertising Sales Manager· Can You Imagine Shooting Animal-Shaped Steel GLENNA A. EIDEN MILLER Targets With A Pistol . .. .That There Is Rifle Range. Advertising Production Director . SPECIAL ..• WINNING TECHNIQUES TOM A. VON ROSEN BULLSEYE, James D.