Do More, Feel Better, Live Longer Annual Report 2012 B GSK Annual Report 2012
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNITED STATES SECURITIES and EXCHANGE COMMISSION Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 SCHEDULE 13D Under the Securities Exchange Act of 1934 (Amendment No. 2)* GENOCEA BIOSCIENCES, INC. (Name of Issuer) Common Stock, Par Value $0.001 (Title of Class of Securities) 372427 10 4 (CUSIP Number) Victoria A. Whyte GlaxoSmithKline plc 980 Great West Road Brentford, Middlesex TW8 9GS England Telephone: +44 (0)208 047 5000 Name, Address and Telephone Number of Person Authorized to Receive Notices and Communications) July 30, 2015 (Date of Event which Requires Filing of this Statement) If the filing person has previously filed a statement on Schedule 13G to report the acquisition that is the subject of this Schedule 13D, and is filing this schedule because of §§240.13d-1(e), 240.13d-1(f) or 240.13d-1(g), check the following box. ☐ Note: Schedules filed in paper format shall include a signed original and five copies of the schedule, including all exhibits. See Rule.13d-7 for other parties to whom copies are to be sent. * The remainder of this cover page shall be filled out for a reporting person’s initial filing on this form with respect to the subject class of securities, and for any subsequent amendment containing information which would alter disclosures provided in a prior cover page. The information required on the remainder of this cover page shall not be deemed to be “filed” for the purpose of Section 18 of the Securities Exchange Act of 1934 (“Act”) or otherwise subject to the liabilities of that section of the Act but shall be subject to all other provisions of the Act (however, see the Notes). -

Corporate Governance
Strategic report Governance and remuneration Financial statements Investor information Corporate Governance In this section Chairman’s Governance statement 78 The Board 80 Corporate Executive Team 83 Board architecture 85 Board roles and responsibilities 86 Board activity and principal decisions 87 Our purpose, values and culture 90 The Board’s approach to engagement 91 Board performance 94 Board Committee information 96 Our Board Committee reports 97 Section 172 statement 108 Directors’ report 109 GSK Annual Report 2020 77 Chairman’s Governance statement In last year’s Governance statement, I explained that our primary Education and focus on Science objective for 2020 was to ensure there was clarity between the Given the critical importance of strengthening the pipeline, Board and management on GSK’s execution of strategy and its the Board has benefitted from devoting a higher proportion of operational priorities. We have aligned our long-term priorities its time in understanding the science behind our strategy and of Innovation, Performance and Trust powered by culture testing its application. It is important that the Board has a and agreed on the metrics to measure delivery against them. working understanding of the key strategic themes upon The Board’s annual cycle of meetings ensures that all major which our R&D strategy is based. These themes have been components of our strategy are reviewed over the course complemented by Board R&D science thematic deep dives. of the year. Our focus was on the fundamentals of our strategy: human The COVID-19 pandemic impacted and dominated all our genetics, the immune system and AI and ML, as well as to lives for the majority of 2020. -

Governance and Remuneration 2014
Governance & remuneration reportStrategic In this section Our Board 72 Our Corporate Executive Team 76 Chairman’s letter 78 Corporate governance framework 79 Board report to shareholders Oversight and stewardship in 2014 and future actions 80 remuneration & Governance Leadership and effectiveness 82 Committee reports Audit & Risk 86 Nominations 92 Corporate Responsibility 94 Remuneration report Chairman’s annual statement 96 Annual report on remuneration 97 2014 Remuneration policy report 119 Financial statements Financial Investor information Investor GSK Annual Report 2014 71 Our Board Strategic reportStrategic Diversity Experience International experience Composition Tenure (Non-Executives) % % % % Scientific 19 Global 75 Executive 19 Up to 3 years 39 % % % % Finance 31 USA 100 Non-Executive 81 3-6 years 15 % % % % Industry 50 Europe 94 Male 69 7-9 years 23 % % % EMAP 63 Female 31 Over 9 years 23 Sir Christopher Gent 66 Skills and experience Chairman Sir Christopher has many years of experience of leading global businesses and a track record of delivering outstanding performance Governance & remuneration & Governance Nationality in highly competitive industries. He was appointed Managing Director British of Vodafone plc in 1985 and then became its Chief Executive Officer Appointment date in 1997 until his retirement in 2003. Sir Christopher was also a 1 June 2004 and as Chairman Non-Executive Director of Ferrari SpA and a member of the British on 1 January 2005 Airways International Business Advisory Board. Committee membership External appointments Corporate Responsibility Sir Christopher is a Senior Adviser at Bain & Co. Committee Chairman, Nominations, Remuneration and Finance Sir Philip Hampton 61 Skills and experience Chairman Designate Prior to joining GSK, Sir Philip chaired major FTSE 100 companies including J Sainsbury plc. -

Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation
www.sciencemag.org/cgi/content/full/327/5963/348/DC1 Supporting Online Material for Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation Jason Rihel,* David A. Prober, Anthony Arvanites, Kelvin Lam, Steven Zimmerman, Sumin Jang, Stephen J. Haggarty, David Kokel, Lee L. Rubin, Randall T. Peterson, Alexander F. Schier* *To whom correspondence should be addressed. E-mail: [email protected] (A.F.S.); [email protected] (J.R.) Published 15 January 2010, Science 327, 348 (2010) DOI: 10.1126/science.1183090 This PDF file includes: Materials and Methods SOM Text Figs. S1 to S18 Table S1 References Supporting Online Material Table of Contents Materials and Methods, pages 2-4 Supplemental Text 1-7, pages 5-10 Text 1. Psychotropic Drug Discovery, page 5 Text 2. Dose, pages 5-6 Text 3. Therapeutic Classes of Drugs Induce Correlated Behaviors, page 6 Text 4. Polypharmacology, pages 6-7 Text 5. Pharmacological Conservation, pages 7-9 Text 6. Non-overlapping Regulation of Rest/Wake States, page 9 Text 7. High Throughput Behavioral Screening in Practice, page 10 Supplemental Figure Legends, pages 11-14 Figure S1. Expanded hierarchical clustering analysis, pages 15-18 Figure S2. Hierarchical and k-means clustering yield similar cluster architectures, page 19 Figure S3. Expanded k-means clustergram, pages 20-23 Figure S4. Behavioral fingerprints are stable across a range of doses, page 24 Figure S5. Compounds that share biological targets have highly correlated behavioral fingerprints, page 25 Figure S6. Examples of compounds that share biological targets and/or structural similarity that give similar behavioral profiles, page 26 Figure S7. -

Annual Report 2013
Annual Report 2013 “ Being active and having a positive outlook on life is what keeps me going every day.” Overview of 2013 “ Our performance in 2013 was defined by remarkable &R D output and further delivery of sustained financial performance for our shareholders.” Please go to page 4 for more More at gsk.com Performance highlights £26.5bn £8.0bn £7.0bn £5.2bn Group turnover Core* operating profit Total operating profit Returned to shareholders 6 112.2p 112.5p 13% Major medicines approved Core* earnings per share Total earnings per share Estimated return on R&D investment 10 6 1st 1st Potential phase III study starts in 2014/15 Potential medicines with phase III data in Access to Medicines Index Pharmaceutical company to sign AllTrials expected 2014/15 campaign for research transparency Front cover story Betty, aged 65, (pictured) has Chronic “ Health is important to me, Obstructive Pulmonary Disease (COPD). She only has 25% lung capacity. This means I try to take care of my she finds even everyday tasks difficult, but medicines and inhaled oxygen allow her to health with all the tools live as normal a life as she can. Betty’s mindset I have and do the best is to stay busy and active, so every week she goes to rehab exercise classes. that I can with it.” COPD is a disease of the lungs that leads to Betty, COPD patient, damaged airways, causing them to become North Carolina, USA narrower and making it harder for air to get in and out. 210 million people around the world are estimated to have COPD. -

Annual Review 2005
GS2184_Review_A\W2.qxd 7/3/06 4:58 pm Page fc1 Annual Review 2005 human being Do more, feel better, live longer GS2184_Review_A\W2.qxd 9/3/06 1:28 pm Page ifc2 01 An interview with Sir Christopher Gent, Chairman, and JP Garnier, Chief Executive Officer 05 Tachi Yamada, Chairman, Research & Development, Pharmaceuticals 06 Jean Stéphenne, President and General Manager, GSK Biologicals 08 John Clarke, President, Consumer Healthcare 11 David Stout, President, Pharmaceutical Operations 14 Performance highlights 15 Business operating review 18 The Board 19 The Corporate Executive Team 20 Summary remuneration report 23 Corporate governance 24 Responsibility statements 25 Summary financial statements 26 Summary information under US GAAP 27 Shareholder information 29 Chairman and CEO’s closing letter JP Garnier (left) and Sir Christopher Gent (right) GS2184_Review_A\W2.qxd 7/3/06 5:01 pm Page 01 “Discovering important medicines, eradicating diseases, improving the quality of people’s lives and making medicines available to a greater number of people. This is what we do – and what we do matters to people.” JP Garnier, Chief Executive Officer An interview with Sir Christopher Gent, Chairman and JP Garnier, Chief Executive Officer 2005: a year of success and progress “Thanks to the efforts of our employees around the company’s pipeline is one of the largest and most world, 2005 was a very successful year for GSK,” says promising in the industry, with 149 projects in clinical JP Garnier, Chief Executive Officer. “Not only was it development (as at the end of February 2006), our best year ever from a financial standpoint, we also including 95 new chemical entities (NCEs), 29 product made substantial progress with our pipeline of line extensions (PLEs) and 25 vaccines. -

Other Statutory Disclosures Continued
Other statutory disclosures continued Strategic reportGroup companies Governance & remuneration Financial statements In accordance with Section 409 of the Companies Act 2006 a full list of subsidiaries, associates, joint ventures and joint arrangements, the country of incorporation and effective percentage of equity owned, as at 31 December 2015 are disclosed below. Unless otherwise stated the share capital disclosed comprises ordinary shares which are indirectly held by GlaxoSmithKline plc. All subsidiary companies are resident for tax purposes in their country of incorporation unless otherwise stated. Country of Effective % % Held by Name incorporation Ownership Security Class of Share Wholly owned subsidiaries 1506369 Alberta ULC Canada 100 Common 100 Action Potential Venture Capital Limited England & Wales 100 Ordinary 100 Adechsa GmbH Switzerland 100 Ordinary 100 Affymax Research Institute United States 100 Common 100 Alenfarma – Especialidades Farmaceuticas, Limitada (iv) Portugal 100 Ordinary Quota 100 Allen & Hanburys Limited (iv) England & Wales 100 Ordinary 100 Allen & Hanburys Pharmaceutical Nigeria Limited Nigeria 100 Ordinary 100 Allen Farmaceutica, S.A. Spain 100 Ordinary 100 Allen Pharmazeutika Gesellschaft m.b.H. Austria 100 Ordinary 100 Aners S.A (iv) Argentina 100 Non-endorsable Nominative Ordinary 100 Barrier Therapeutics, Inc. United States 100 Common 100 Beecham Group p l c England & Wales 100 20p Shares 'A'; 5p Shares B 100 Beecham Pharmaceuticals (Pte) Limited Singapore 100 Ordinary 100 Beecham Pharmaceuticals S.A (iv) (vi) Ecuador 100 Nominative 100 Beecham Portuguesa-Produtos Farmaceuticos e Quimicos, Lda Portugal 100 Ordinary Quota 100 Beecham S.A. (iv) Belgium 100 Ordinary 100 Biddle Sawyer Limited India 100 Equity 100 Biovesta Ilaçlari Ltd. Sti. Turkey 100 Nominative 100 Burroughs Wellcome & Co (Australia) Pty Limited (iv) (vi) Australia 100 Ordinary 100 Burroughs Wellcome & Co (Bangladesh) Limited Bangladesh 100 Ordinary 100 Burroughs Wellcome International Limited England & Wales 100 Ordinary 100 Caribbean Chemical Company, Ltd. -

Clinical Guidelines for Use of Depot Buprenorphine (Buvidal®
Clinical guidelines for use of depot buprenorphine (Buvidal® and Sublocade®) in the treatment of opioid dependence NSW Ministry of Health 100 Christie Street ST LEONARDS NSW 2065 Tel. (02) 9391 9000 Fax. (02) 9391 9101 TTY. (02) 9391 9900 www.health.nsw.gov.au Produced by: NSW Ministry of Health Lintzeris N, Dunlop A, Masters D (2019) Clinical guidelines for use of depot buprenorphine (Buvidal® and Sublocade®) in the treatment of opioid dependence. NSW Ministry of Health, Sydney Australia This work is copyright. It may be reproduced in whole or in part for study or training purposes subject to the inclusion of an acknowledgement of the source. It may not be reproduced for commercial usage or sale. Reproduction for purposes other than those indicated above requires written permission from the NSW Ministry of Health. © NSW Ministry of Health 2019 SHPN (CPH) 190431 ISBN 978-1-76081-226-3 Further copies of this document can be downloaded from the NSW Health website www.health.nsw.gov.au August 2019 ii NSW Health Use of depot BPN in the treatment of opioid dependence Authors Professor Nicholas Lintzeris (BMedSci MBBS PhD FAChAM) Director Drug and Alcohol Services, South East Sydney Local Health District Discipline Addiction Medicine, Faculty of Medicine and Health Sciences, University of Sydney NSW Drug and Alcohol Clinical Research and Improvement Network Professor Adrian Dunlop (MBBS, PhD, GdipEpiBiotat, FAChAM) Director Drug and Alcohol Clinical Services, Hunter New England Local Health District School of Medicine and Public Health, Faculty -

In This Section
Strategic report In this section Chairman’s statement 2 CEO’s review 4 Business overview 6 The global context 8 Our business model 12 Our strategic priorities 14 How we performed 16 Risk management 18 Grow 20 Deliver 32 Simplify 44 Our financial architecture 48 Responsible business 50 Financial review 58 Strategic report Chairman’s statement Chairman’s statement To shareholders The value of the significant changes that have been made in recent years is evidenced in our performance this year “ Since Sir Andrew became It is clear from the following pages that Through the Audit & Risk Committee, we the Group made good progress against oversee the issues and challenges faced by CEO, the company has its strategy in 2013. management, and encourage the creation of an environment in which GSK can achieve The Board believes the business is seeing returned £30 billion its strategic ambitions in a responsible and the benefits of the significant changes the sustainable manner. to shareholders.” management team has driven over recent years to deliver sustainable growth, reduce risk and I have no doubt that commercial success is enhance returns to shareholders. directly linked to operating in a responsible way and which meets the changing expectations of The notably strong performance from the society. In this respect, the company continues R&D organisation in 2013 – with six major to adopt industry-leading positions on a range new product approvals in areas including of issues. respiratory disease, HIV and cancer – is critical to the longer-term prospects of the The announcement of plans during 2013 to Group. -
Voting/Poll Results
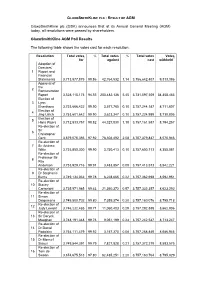
GLAXOSMITHKLINE PLC - RESULT OF AGM GlaxoSmithKline plc (GSK) announces that at its Annual General Meeting (AGM) today, all resolutions were passed by shareholders. GlaxoSmithKline AGM Poll Results The following table shows the votes cast for each resolution: Resolution Total votes % Total votes % Total votes Votes for* against cast withheld** Adoption of Directors’ 1 Report and Financial Statements 3,713,877,875 98.86 42,764,532 1.14 3,756,642,407 9,313,386 Approval of the 2 Remuneration Report 3,528,115,173 94.55 203,482,136 5.45 3,731,597,309 34,358,483 Election of 3 Lynn Elsenhans 3,753,666,422 99.90 3,577,765 0.10 3,757,244,187 8,711,607 Election of 4 Jing Ulrich 3,753,601,642 99.90 3,623,347 0.10 3,757,224,989 8,730,805 Election of 5 Hans Wijers 3,712,833,757 98.82 44,327,830 1.18 3,757,161,587 8,794,257 Re-election of Sir 6 Christopher Gent 3,679,075,355 97.92 78,304,492 2.08 3,757,379,847 8,575,946 Re-election of 7 Sir Andrew Witty 3,753,850,300 99.90 3,750,413 0.10 3,757,600,713 8,355,081 Re-election of Professor Sir 8 Roy Anderson 3,753,929,716 99.91 3,483,857 0.09 3,757,413,573 8,542,221 Re-election of 9 Dr Stephanie Burns 3,749,134,033 99.78 8,228,665 0.22 3,757,362,698 8,592,951 Re-election of 10 Stacey Cartwright 3,735,971,985 99.43 21,360,372 0.57 3,757,332,357 8,623,292 Re-election of 11 Simon Dingemans 3,749,800,702 99.80 7,359,374 0.20 3,757,160,076 8,795,718 Re-election of 12 Judy Lewent 3,746,232,485 99.71 11,060,403 0.29 3,757,292,888 8,662,906 Re-election of 13 Sir Deryck Maughan 3,748,191,348 99.76 9,051,199 0.24 -

Glaxosmithkline Plc Annual Report for the Year Ended 31St December 2000
GlaxoSmithKline 01 GlaxoSmithKline plc Annual Report for the year ended 31st December 2000 Contents Report of the Directors 02 Financial summary 03 Joint statement by the Chairman and the Chief Executive Officer 05 Description of business 29 Corporate governance 37 Remuneration report 47 Operating and financial review and prospects 69 Financial statements 70 Directors’ statements of responsibility 71 Report by the auditors 72 Consolidated statement of profit and loss 72 Consolidated statement of total recognised gains and losses 74 Consolidated statement of cash flow 76 Consolidated balance sheet 76 Reconciliation of movements in equity shareholders’ funds 77 Company balance sheet 78 Notes to the financial statements 136 Group companies 142 Principal financial statements in US$ 144 Financial record 153 Investor information 154 Shareholder return 156 Taxation information for shareholders 157 Shareholder information 158 Share capital 160 Cross reference to Form 20-F 162 Glossary of terms The Annual Report was approved by the Board 163 Index of Directors on 22nd March 2001 and published on 12th April 2001. Contact details 02 GlaxoSmithKline Financial summary 2000 1999 Increase Business performance £m £m CER % £ % Sales 18,079 16,164 9 12 Trading profit 5,026 4,378 12 15 Profit before taxation 5,327 4,708 11 13 Earnings/Net income 3,697 3,222 13 15 Earnings per Ordinary Share 61.0p 52.7p 14 16 Total results Profit before taxation 6,029 4,236 Earnings/Net income 4,154 2,859 Earnings per Ordinary Share 68.5p 46.7p Business performance: results exclude merger items and restructuring costs; 1999 sales and trading profit exclude the Healthcare Services businesses which were disposed of in 1999. -

Oak Associates Funds Annual Proxy Voting Record
FilePoint® Form Type: N-PX Period: 06-30-2020 Sequence: 1 Document Name: fp0056277_npx.htm UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM N-PX ANNUAL REPORT OF PROXY VOTING RECORD OF REGISTERED MANAGEMENT INVESTMENT COMPANY Investment Company Act file number: 811-08549 OAK ASSOCIATES FUNDS (Exact name of registrant as specified in charter) 3875 Embassy Parkway, Suite 250, Akron, Ohio 44333-8334 (Address of principal executive offices) (Zip code) Charles A. Kiraly Oak Associates Funds 3875 Embassy Parkway, Suite 250 Akron, Ohio 44333-8334 (Name and address of agent for service) Registrant’s Telephone Number, including Area Code: (888) 462-5386 Date of fiscal year end: October 31 Date of reporting period: July 1, 2019 - June 30, 2020 FilePoint® Form Type: N-PX Period: 06-30-2020 Sequence: 2 Document Name: fp0056277_npx.htm Item 1. Proxy Voting Record. ******************************* FORM N-Px REPORT ******************************* ICA File Number: 811-08549 Reporting Period: 07/01/2019 - 06/30/2020 Oak Associates Funds ====================== Black Oak Emerging Technology Fund ====================== ADVANCED ENERGY INDUSTRIES, INC. Ticker: AEIS Security ID: 007973100 Meeting Date: APR 30, 2020 Meeting Type: Annual Record Date: MAR 09, 2020 # Proposal Mgt Rec Vote Cast Sponsor 1.1 Elect Director Grant H. Beard For For Management 1.2 Elect Director Frederick A. Ball For For Management 1.3 Elect Director Tina M. Donikowski For For Management 1.4 Elect Director Ronald C. Foster For For Management 1.5 Elect Director Edward C. Grady For For Management 1.6 Elect Director Thomas M. Rohrs For For Management 1.7 Elect Director John A.