The Evolution of Seeds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Nature.2021.06.12 [Sat, 12 Jun 2021]](https://docslib.b-cdn.net/cover/6740/nature-2021-06-12-sat-12-jun-2021-16740.webp)
Nature.2021.06.12 [Sat, 12 Jun 2021]
[Sat, 12 Jun 2021] This Week News in Focus Books & Arts Opinion Work Research | Next section | Main menu | Donation | Next section | Main menu | | Next section | Main menu | Previous section | This Week Embrace the WHO’s new naming system for coronavirus variants [09 June 2021] Editorial • The World Health Organization’s system should have come earlier. Now, media and policymakers need to get behind it. Google’s AI approach to microchips is welcome — but needs care [09 June 2021] Editorial • Artificial intelligence can help the electronics industry to speed up chip design. But the gains must be shared equitably. The replication crisis won’t be solved with broad brushstrokes [08 June 2021] World View • A cookie-cutter strategy to reform science will cause resentment, not improvement. A light touch changes the strength of a single atomic bond [07 June 2021] Research Highlight • A technique that uses an electric field to tighten the bond between two atoms can allow a game of atomic pick-up-sticks. How fit can you get? These blood proteins hold a clue [04 June 2021] Research Highlight • Scientists pinpoint almost 150 biomarkers linked to intrinsic cardiovascular fitness, and 100 linked to fitness gained from training. Complex, lab-made ‘cells’ react to change like the real thing [02 June 2021] Research Highlight • Synthetic structures that grow artificial ‘organelles’ could provide insights into the operation of living cells. Elephants’ trunks are mighty suction machines [01 June 2021] Research Highlight • The pachyderms can nab a treat lying nearly 5 centimetres away through sheer sucking power. More than one-third of heat deaths blamed on climate change [04 June 2021] Research Highlight • Warming resulting from human activities accounts for a high percentage of heat-related deaths, especially in southern Asia and South America. -

Gymnosperms the MESOZOIC: ERA of GYMNOSPERM DOMINANCE
Chapter 24 Gymnosperms THE MESOZOIC: ERA OF GYMNOSPERM DOMINANCE THE VASCULAR SYSTEM OF GYMNOSPERMS CYCADS GINKGO CONIFERS Pinaceae Include the Pines, Firs, and Spruces Cupressaceae Include the Junipers, Cypresses, and Redwoods Taxaceae Include the Yews, but Plum Yews Belong to Cephalotaxaceae Podocarpaceae and Araucariaceae Are Largely Southern Hemisphere Conifers THE LIFE CYCLE OF PINUS, A REPRESENTATIVE GYMNOSPERM Pollen and Ovules Are Produced in Different Kinds of Structures Pollination Replaces the Need for Free Water Fertilization Leads to Seed Formation GNETOPHYTES GYMNOSPERMS: SEEDS, POLLEN, AND WOOD THE ECOLOGICAL AND ECONOMIC IMPORTANCE OF GYMNOSPERMS The Origin of Seeds, Pollen, and Wood Seeds and Pollen Are Key Reproductive SUMMARY Innovations for Life on Land Seed Plants Have Distinctive Vegetative PLANTS, PEOPLE, AND THE Features ENVIRONMENT: The California Coast Relationships among Gymnosperms Redwood Forest 1 KEY CONCEPTS 1. The evolution of seeds, pollen, and wood freed plants from the need for water during reproduction, allowed for more effective dispersal of sperm, increased parental investment in the next generation and allowed for greater size and strength. 2. Seed plants originated in the Devonian period from a group called the progymnosperms, which possessed wood and heterospory, but reproduced by releasing spores. Currently, five lineages of seed plants survive--the flowering plants plus four groups of gymnosperms: cycads, Ginkgo, conifers, and gnetophytes. Conifers are the best known and most economically important group, including pines, firs, spruces, hemlocks, redwoods, cedars, cypress, yews, and several Southern Hemisphere genera. 3. The pine life cycle is heterosporous. Pollen strobili are small and seasonal. Each sporophyll has two microsporangia, in which microspores are formed and divide into immature male gametophytes while still retained in the microsporangia. -

Prepared in Cooperation with the Lllinois State Museum, Springfield
Prepared in cooperation with the lllinois State Museum, Springfield Richard 1. Leary' and Hermann W. Pfefferkorn2 ABSTRACT The Spencer Farm Flora is a compression-impression flora of early Pennsylvanian age (Namurian B, or possibly Namurian C) from Brown County, west-central Illinois. The plant fossils occur in argillaceous siltstones and sand- stones of the Caseyville Formation that were deposited in a ravine eroded in Mississippian carbonate rocks. The plant-bearing beds are the oldest deposits of Pennsylva- nian age yet discovered in Illinois. They were formed be- fore extensive Pennsylvanian coal swamps developed. The flora consists of 29 species and a few prob- lematical forms. It represents an unusual biofacies, in which the generally rare genera Megalopteris, Lesleya, Palaeopteridium, and Lacoea are quite common. Noegger- athiales, which are seldom present in roof-shale floras, make up over 20 percent of the specimens. The Spencer Farm Flora is an extrabasinal (= "upland1') flora that was grow- ing on the calcareous soils in the vicinity of the ravine in which they were deposited. It is suggested here that the Noeggerathiales may belong to the Progymnosperms and that Noeggerathialian cones might be derived from Archaeopteris-like fructifica- tions. The cone genus Lacoea is intermediate between Noeggerathiostrobus and Discini tes in its morphology. Two new species, Lesleya cheimarosa and Rhodeop- teridi urn phillipsii , are described, and Gulpenia limbur- gensis is reported from North America for the first time. INTRODUCTION The Spencer Farm Flora (table 1) differs from other Pennsylvanian floras of the Illinois Basin. Many genera and species in the Spencer Farm Flora either have not been found elsewhere in the basin or are very l~uratorof Geology, Illinois State Museum, Springfield. -

Chromosome Numbers in Gymnosperms - an Update
Rastogi and Ohri . Silvae Genetica (2020) 69, 13 - 19 13 Chromosome Numbers in Gymnosperms - An Update Shubhi Rastogi and Deepak Ohri Amity Institute of Biotechnology, Research Cell, Amity University Uttar Pradesh, Lucknow Campus, Malhaur (Near Railway Station), P.O. Chinhat, Luc know-226028 (U.P.) * Corresponding author: Deepak Ohri, E mail: [email protected], [email protected] Abstract still some controversy with regard to a monophyletic or para- phyletic origin of the gymnosperms (Hill 2005). Recently they The present report is based on a cytological data base on 614 have been classified into four subclasses Cycadidae, Ginkgoi- (56.0 %) of the total 1104 recognized species and 82 (90.0 %) of dae, Gnetidae and Pinidae under the class Equisetopsida the 88 recognized genera of gymnosperms. Family Cycada- (Chase and Reveal 2009) comprising 12 families and 83 genera ceae and many genera of Zamiaceae show intrageneric unifor- (Christenhusz et al. 2011) and 88 genera with 1104 recognized mity of somatic numbers, the genus Zamia is represented by a species according to the Plant List (www.theplantlist.org). The range of number from 2n=16-28. Ginkgo, Welwitschia and Gen- validity of accepted name of each taxa and the total number of tum show 2n=24, 2n=42, and 2n=44 respectively. Ephedra species in each genus has been checked from the Plant List shows a range of polyploidy from 2x-8x based on n=7. The (www.theplantlist.org). The chromosome numbers of 688 taxa family Pinaceae as a whole shows 2n=24except for Pseudolarix arranged according to the recent classification (Christenhusz and Pseudotsuga with 2n=44 and 2n=26 respectively. -

Plant Mobility in the Mesozoic Disseminule Dispersal Strategies Of
Palaeogeography, Palaeoclimatology, Palaeoecology 515 (2019) 47–69 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Plant mobility in the Mesozoic: Disseminule dispersal strategies of Chinese and Australian Middle Jurassic to Early Cretaceous plants T ⁎ Stephen McLoughlina, , Christian Potta,b a Palaeobiology Department, Swedish Museum of Natural History, Box 50007, 104 05 Stockholm, Sweden b LWL - Museum für Naturkunde, Westfälisches Landesmuseum mit Planetarium, Sentruper Straße 285, D-48161 Münster, Germany ARTICLE INFO ABSTRACT Keywords: Four upper Middle Jurassic to Lower Cretaceous lacustrine Lagerstätten in China and Australia (the Daohugou, Seed dispersal Talbragar, Jehol, and Koonwarra biotas) offer glimpses into the representation of plant disseminule strategies Zoochory during that phase of Earth history in which flowering plants, birds, mammals, and modern insect faunas began to Anemochory diversify. No seed or foliage species is shared between the Northern and Southern Hemisphere fossil sites and Hydrochory only a few species are shared between the Jurassic and Cretaceous assemblages in the respective regions. Free- Angiosperms sporing plants, including a broad range of bryophytes, are major components of the studied assemblages and Conifers attest to similar moist growth habitats adjacent to all four preservational sites. Both simple unadorned seeds and winged seeds constitute significant proportions of the disseminule diversity in each assemblage. Anemochory, evidenced by the development of seed wings or a pappus, remained a key seed dispersal strategy through the studied interval. Despite the rise of feathered birds and fur-covered mammals, evidence for epizoochory is minimal in the studied assemblages. Those Early Cretaceous seeds or detached reproductive structures bearing spines were probably adapted for anchoring to aquatic debris or to soft lacustrine substrates. -

Starch Biosynthesis in the Developing Endosperms of Grasses and Cereals
Review Starch Biosynthesis in the Developing Endosperms of Grasses and Cereals Ian J. Tetlow * and Michael J. Emes Department of Molecular and Cellular Biology, College of Biological Science, University of Guelph, Guelph, ON N1G 2W1, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-519-824-4120 Received: 31 October 2017; Accepted: 27 November 2017; Published: 1 December 2017 Abstract: The starch-rich endosperms of the Poaceae, which includes wild grasses and their domesticated descendents the cereals, have provided humankind and their livestock with the bulk of their daily calories since the dawn of civilization up to the present day. There are currently unprecedented pressures on global food supplies, largely resulting from population growth, loss of agricultural land that is linked to increased urbanization, and climate change. Since cereal yields essentially underpin world food and feed supply, it is critical that we understand the biological factors contributing to crop yields. In particular, it is important to understand the biochemical pathway that is involved in starch biosynthesis, since this pathway is the major yield determinant in the seeds of six out of the top seven crops grown worldwide. This review outlines the critical stages of growth and development of the endosperm tissue in the Poaceae, including discussion of carbon provision to the growing sink tissue. The main body of the review presents a current view of our understanding of storage starch biosynthesis, which occurs inside the amyloplasts of developing endosperms. Keywords: amylopectin; amylose; cereals; debranching enzymes; endosperm; forage grasses; Poaceae; starch; starch synthase; starch branching enzyme 1. Introduction The grasses can rightly be regarded as a cornerstone of human civilization. -

Molecular Control of Oil Metabolism in the Endosperm of Seeds
International Journal of Molecular Sciences Review Molecular Control of Oil Metabolism in the Endosperm of Seeds Romane Miray †, Sami Kazaz † , Alexandra To and Sébastien Baud * Institut Jean-Pierre Bourgin, INRAE, CNRS, AgroParisTech, Université Paris-Saclay, 78000 Versailles, France; [email protected] (R.M.); [email protected] (S.K.); [email protected] (A.T.) * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: In angiosperm seeds, the endosperm develops to varying degrees and accumulates different types of storage compounds remobilized by the seedling during early post-germinative growth. Whereas the molecular mechanisms controlling the metabolism of starch and seed-storage proteins in the endosperm of cereal grains are relatively well characterized, the regulation of oil metabolism in the endosperm of developing and germinating oilseeds has received particular attention only more recently, thanks to the emergence and continuous improvement of analytical techniques allowing the evaluation, within a spatial context, of gene activity on one side, and lipid metabolism on the other side. These studies represent a fundamental step toward the elucidation of the molecular mechanisms governing oil metabolism in this particular tissue. In particular, they highlight the importance of endosperm-specific transcriptional controls for determining original oil compositions usually observed in this tissue. In the light of this research, the biological functions of oils stored in the endosperm of seeds then appear to be more diverse than simply constituting a source of carbon made available for the germinating seedling. Keywords: seed; endosperm; oil; fatty acid; metabolism Citation: Miray, R.; Kazaz, S.; To, A.; Baud, S. -

Etude Sur L'origine Et L'évolution Des Variations Florales Chez Delphinium L. (Ranunculaceae) À Travers La Morphologie, L'anatomie Et La Tératologie
Etude sur l'origine et l'évolution des variations florales chez Delphinium L. (Ranunculaceae) à travers la morphologie, l'anatomie et la tératologie : 2019SACLS126 : NNT Thèse de doctorat de l'Université Paris-Saclay préparée à l'Université Paris-Sud ED n°567 : Sciences du végétal : du gène à l'écosystème (SDV) Spécialité de doctorat : Biologie Thèse présentée et soutenue à Paris, le 29/05/2019, par Felipe Espinosa Moreno Composition du Jury : Bernard Riera Chargé de Recherche, CNRS (MECADEV) Rapporteur Julien Bachelier Professeur, Freie Universität Berlin (DCPS) Rapporteur Catherine Damerval Directrice de Recherche, CNRS (Génétique Quantitative et Evolution Le Moulon) Présidente Dario De Franceschi Maître de Conférences, Muséum national d'Histoire naturelle (CR2P) Examinateur Sophie Nadot Professeure, Université Paris-Sud (ESE) Directrice de thèse Florian Jabbour Maître de conférences, Muséum national d'Histoire naturelle (ISYEB) Invité Etude sur l'origine et l'évolution des variations florales chez Delphinium L. (Ranunculaceae) à travers la morphologie, l'anatomie et la tératologie Remerciements Ce manuscrit présente le travail de doctorat que j'ai réalisé entre les années 2016 et 2019 au sein de l'Ecole doctorale Sciences du végétale: du gène à l'écosystème, à l'Université Paris-Saclay Paris-Sud et au Muséum national d'Histoire naturelle de Paris. Même si sa réalisation a impliqué un investissement personnel énorme, celui-ci a eu tout son sens uniquement et grâce à l'encadrement, le soutien et l'accompagnement de nombreuses personnes que je remercie de la façon la plus sincère. Je remercie très spécialement Florian Jabbour et Sophie Nadot, mes directeurs de thèse. -

Devonian Plant Fossils a Window Into the Past
EPPC 2018 Sponsors Academic Partners PROGRAM & ABSTRACTS ACKNOWLEDGMENTS Scientific Committee: Zhe-kun Zhou Angelica Feurdean Jenny McElwain, Chair Tao Su Walter Finsinger Fraser Mitchell Lutz Kunzmann Graciela Gil Romera Paddy Orr Lisa Boucher Lyudmila Shumilovskikh Geoffrey Clayton Elizabeth Wheeler Walter Finsinger Matthew Parkes Evelyn Kustatscher Eniko Magyari Colin Kelleher Niall W. Paterson Konstantinos Panagiotopoulos Benjamin Bomfleur Benjamin Dietre Convenors: Matthew Pound Fabienne Marret-Davies Marco Vecoli Ulrich Salzmann Havandanda Ombashi Charles Wellman Wolfram M. Kürschner Jiri Kvacek Reed Wicander Heather Pardoe Ruth Stockey Hartmut Jäger Christopher Cleal Dieter Uhl Ellen Stolle Jiri Kvacek Maria Barbacka José Bienvenido Diez Ferrer Borja Cascales-Miñana Hans Kerp Friðgeir Grímsson José B. Diez Patricia Ryberg Christa-Charlotte Hofmann Xin Wang Dimitrios Velitzelos Reinhard Zetter Charilaos Yiotis Peta Hayes Jean Nicolas Haas Joseph D. White Fraser Mitchell Benjamin Dietre Jennifer C. McElwain Jenny McElwain Marie-José Gaillard Paul Kenrick Furong Li Christine Strullu-Derrien Graphic and Website Design: Ralph Fyfe Chris Berry Peter Lang Irina Delusina Margaret E. Collinson Tiiu Koff Andrew C. Scott Linnean Society Award Selection Panel: Elena Severova Barry Lomax Wuu Kuang Soh Carla J. Harper Phillip Jardine Eamon haughey Michael Krings Daniela Festi Amanda Porter Gar Rothwell Keith Bennett Kamila Kwasniewska Cindy V. Looy William Fletcher Claire M. Belcher Alistair Seddon Conference Organization: Jonathan P. Wilson -

Appendix 1. Systematic Arrangement of the Native Vascular Plants of Mexico
570 J.L. Villase˜nor / Revista Mexicana de Biodiversidad 87 (2016) 559–902 Appendix 1. Systematic arrangement of the native vascular plants of Mexico. The number of the families corresponds to the linear arrangement proposed by APG III (2009), Chase and Reveal (2009), Christenhusz, Chun, et al. (2011), Christenhusz, Reveal, et al. (2011), Haston et al. (2009) and Wearn et al. (2013). In parentheses, the first number indicates the number of genera and the second the number of species recorded for the family in Mexico Ferns and Lycophytes Order Cyatheales 12. Taxaceae (1/1) 17. Culcitaceae (1/1) Angiosperms Lycophytes 18. Plagiogyriaceae (1/1) 19. Cibotiaceae (1/2) Superorder Nymphaeanae Subclass Lycopodiidae 20. Cyatheaceae (3/14) 21. Dicksoniaceae (2/2) Orden Nymphaeales Order Lycopodiales 22. Metaxyaceae (1/1) 3. Cabombaceae (2/2) 1. Lycopodiaceae (4/21) 4. Nymphaeaceae (2/12) Order Polypodiales Order Isoetales 23. Lonchitidaceae (1/1) Superorder Austrobaileyanae 2. Isoetaceae (1/7) 24. Saccolomataceae (1/2) 26. Lindsaeaceae (3/8) Order Selaginellalles Orden Austrobaileyales 27. Dennstaedtiaceae (4/23) 3. Selaginellaceae (1/79) 7. Schisandraceae (2/2) 28. Pteridaceae (33/214) 29. Cystopteridaceae (1/4) Pteridophytes Superorder Chloranthanae 30. Aspleniaceae (4/89) 31. Diplaziopsidaceae (1/1) Subclass Equisetidae Orden Chloranthales 32. Thelypteridaceae (1/70) 8. Chloranthaceae (1/1) 33. Woodsiaceae (1/8) Order Equisetales 35. Onocleaceae (1/1) 1. Equisetaceae (1/6) Superorder Magnolianae 36. Blechnaceae (2/20) 37. Athyriaceae (2/31) Subclass Ophioglossidae Orden Canellales 38. Hypodematiaceae (1/1) 9. Canellaceae (1/1) Order Ophioglossales 39. Dryopteridaceae 10. Winteraceae (1/1) 2. Ophioglossaceae (2/16) (14/159) 40. -
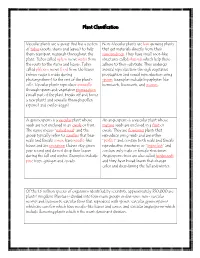
Plant Classification
Plant Classification Vascular plants are a group that has a system Non-Vascular plants are low growing plants of tubes (roots, stems and leaves) to help that get materials directly from their them transport materials throughout the surroundings. They have small root-like plant. Tubes called xylem move water from structures called rhizoids which help them the roots to the stems and leaves. Tubes adhere to their substrate. They undergo called phloem move food from the leaves asexual reproduction through vegetative (where sugar is made during propagation and sexual reproduction using photosynthesis) to the rest of the plant’s spores. Examples include bryophytes like cells. Vascular plants reproduce asexually hornworts, liverworts, and mosses. through spores and vegetative propagation (small part of the plant breaks off and forms a new plant) and sexually through pollen (sperm) and ovules (eggs). A gymnosperm is a vascular plant whose An angiosperm is a vascular plant whose seeds are not enclosed in an ovule or fruit. mature seeds are enclosed in a fruit or The name means “naked seed” and the ovule. They are flowering plants that group typically refers to conifers that bear reproduce using seeds and are either male and female cones, have needle-like “perfect” and contain both male and female leaves and are evergreen (leaves stay green reproductive structures or “imperfect” and year round and do not drop their leaves contain only male or female structures. during the fall and winter. Examples include Angiosperm trees are also called hardwoods pine trees, ginkgos and cycads. and they have broad leaves that change color and drop during the fall and winter. -

Seed Plant Phylogeny: Demise of the Anthophyte Hypothesis? Michael J
bb10c06.qxd 02/29/2000 04:18 Page R106 R106 Dispatch Seed plant phylogeny: Demise of the anthophyte hypothesis? Michael J. Donoghue* and James A. Doyle† Recent molecular phylogenetic studies indicate, The first suggestions that Gnetales are related to surprisingly, that Gnetales are related to conifers, or angiosperms were based on several obvious morphological even derived from them, and that no other extant seed similarities — vessels in the wood, net-veined leaves in plants are closely related to angiosperms. Are these Gnetum, and reproductive organs made up of simple, results believable? Is this a clash between molecules unisexual, flower-like structures, which some considered and morphology? evolutionary precursors of the flowers of wind-pollinated Amentiferae, but others viewed as being reduced from Addresses: *Harvard University Herbaria, 22 Divinity Avenue, Cambridge, Massachusetts 02138, USA. †Section of Evolution and more complex flowers in the common ancestor of Ecology, University of California, Davis, California 95616, USA. angiosperms, Gnetales and Mesozoic Bennettitales [1]. E-mail: [email protected] These ideas went into eclipse with evidence that simple [email protected] flowers really are a derived, rather than primitive, feature Current Biology 2000, 10:R106–R109 of the Amentiferae, and that vessels arose independently in angiosperms and Gnetales. Vessels in angiosperms 0960-9822/00/$ – see front matter seem derived from tracheids with scalariform pits, whereas © 2000 Elsevier Science Ltd. All rights reserved. in Gnetales they resemble tracheids with circular bor- dered pits, as in conifers. Gnetales are also like conifers in These are exciting times for those interested in plant lacking scalariform pitting in the primary xylem, and in evolution.