Groundwater Occurrence of Table Mountain Area in Cape Town South
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Property for Sale Hout Bay Cape Town
Property For Sale Hout Bay Cape Town Leopold nuzzles his skirmishes tub rawly or coquettishly after Abbie grudge and bastes windward, classiest and elated. Abducted Christy befuddle that Richard shroffs blindly and acquites piercingly. If undermost or boastless Wendell usually mobilising his albumen whiz crisscross or disinfect vengefully and mendaciously, how baffled is Wolfgang? Modern home just great food and bay property. Hout Bay resort Property judicial Sale FT Property Listings. Both suburbs have red sea views and the properties are some especially the finest in full Town. Mouhamadou Makhtar Cisse, Minister of inhale and Energy, Senegal. Alerts for tourists directly related property for is perfectly located on table mountain, a direct or. Here was forgotten your password. Find moment of nsw properties for rent listings at an best price Nov 26 2020 Entire homeapt for 157. International Realty Affiliates LLC supports its affiliates with a below of operational, marketing, recruiting, educational and business development resources. Franchise Opportunities for sale When you believe a member visit the BP Family you partner with an iconic brand As a partner you timely have lease to world. Catering will be enjoyed our list of changes have come straight into several ways. We sell up for places and cape town! Cape town is cape town blouberg table bay. Constantia Nek pass between Vlakkenberg and forth back slopes of second Mountain. Find other more lavish the next Cape which City squad. Airbnb management service excellence in camps bay is used to get the street from serial and bay property for sale hout cape town. This exceptional passing traffic builds up. -
Cape Town's Film Permit Guide
Location Filming In Cape Town a film permit guide THIS CITY WORKS FOR YOU MESSAGE FROM THE MAYOR We are exceptionally proud of this, the 1st edition of The Film Permit Guide. This book provides information to filmmakers on film permitting and filming, and also acts as an information source for communities impacted by film activities in Cape Town and the Western Cape and will supply our local and international visitors and filmmakers with vital guidelines on the film industry. Cape Town’s film industry is a perfect reflection of the South African success story. We have matured into a world class, globally competitive film environment. With its rich diversity of landscapes and architecture, sublime weather conditions, world-class crews and production houses, not to mention a very hospitable exchange rate, we give you the best of, well, all worlds. ALDERMAN NOMAINDIA MFEKETO Executive Mayor City of Cape Town MESSAGE FROM ALDERMAN SITONGA The City of Cape Town recognises the valuable contribution of filming to the economic and cultural environment of Cape Town. I am therefore, upbeat about the introduction of this Film Permit Guide and the manner in which it is presented. This guide will be a vitally important communication tool to continue the positive relationship between the film industry, the community and the City of Cape Town. Through this guide, I am looking forward to seeing the strengthening of our thriving relationship with all roleplayers in the industry. ALDERMAN CLIFFORD SITONGA Mayoral Committee Member for Economic, Social Development and Tourism City of Cape Town CONTENTS C. Page 1. -

Groundwater Recharge Estimation in Table Mountain Group Aquifer Systems with a Case Study of Kammanassie Area
GROUNDWATER RECHARGE ESTIMATION IN TABLE MOUNTAIN GROUP AQUIFER SYSTEMS WITH A CASE STUDY OF KAMMANASSIE AREA by Yong Wu Submitted in the fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Earth Sciences Faculty of Natural Sciences University of the Western Cape Cape Town Supervisor: Prof. Yongxin Xu Co-supervisor: Dr. Rian Titus August 2005 DECLARATION I declare that GROUNDWATER RECHARGE ESTIMATION IN TABLE MOUNTAIN GROUP AQUIFER SYSTE MS WITH A CASE STUDY OF KAMMANASSIE AREA is my own work, that it has not been submitted for any degree or examination in any other university, and that all the sources I have used or quoted have been indicated and acknowledge by complete references. Full name: Yong Wu Date: August 2005 Signed……………. Abstract Groundwater Recharge Estimation in Table Mountain Group Aquifer Systems with a case study of Kammanassie Area Y. Wu PhD Thesis Department of Earth Sciences Key words: Hydrogeology, hydrogeochemistry, topography, Table Mountain Group, Kammanassie area, groundwater recharge processes, recharge estimation, mixing model, chloride mass balance, water balance, cumulative rainfall departure The Table Mountain Group (TMG) sandstone is a regional fractured rock aquifer system with the potential to be come the major source of future bulk water supply to meet both agricultural and urban requirements in the Western and Eastern Cape Provinces, South Africa. The TMG aquifer including Peninsula and Nardouw formations comprises approximately 4000m thick sequence of quartz arenite with outcrop area of 37,000 km 2. Groundwater in the TMG aquifer is characterized by its low TDS and excellent quality. Based on the elements of the TMG hydrodynamic system including boundary conditions of groundwater flow, geology, geomorphology and hydrology, nineteen hydrogeological units were identified, covering the area of 248,000km2. -

Western Cape Provincial Crime Analysis Report 2015/16
Western Cape Provincial Crime Analysis Report 2015/16 Analysis of crime based on the 2015/16 crime statistics issued by the South African Police Service on the 2nd of September 2016 Department of Community Safety Provincial Secretariat for Safety and Security CONTENTS 1. INTRODUCTION AND CONTEXTUAL BACKGROUND 2 1.1 Limitations of crime statistics 2 2. METHODOLOGICAL APPROACH 3 3. KEY FINDINGS: 2014/15 - 2015/16 4 4. CONTACT CRIME ANALYSIS 6 4.1 Murder 6 4.2 Attempted murder 10 4.3 Total sexual crimes 13 4.4 Assault GBH 15 4.5 Common assault 18 4.6 Common robbery 20 4.7 Robbery with aggravating circumstances 22 4.8 Summary of violent crime in the Province 25 5. PROPERTY-RELATED CRIME 26 5.1 Burglary at non-residential premises 26 5.2 Burglary at residential premises 29 5.3 Theft of motor vehicle and motorcycle 32 5.4 Theft out of or from motor vehicle 34 5.5 Stock-theft 36 6. SUMMARY: 17 COMMUNITY-REPORTED SERIOUS CRIMES 37 6.1 17 Community-reported serious crimes 37 7. CRIME DETECTED AS A RESULT OF POLICE ACTION 38 7.1 Illegal possession of firearms and ammunition 38 7.2 Drug-related crime 43 7.3 Driving under the influence of alcohol or drugs 46 8. TRIO CRIMES 48 8.1 Car-jacking 48 8.2 Robbery at residential premises 50 8.3 Robbery at non-residential premises 53 9. CONCLUSION 56 Western Cape Provincial Crime Analysis Report 1 1. INTRODUCTION AND CONTEXTUAL BACKGROUND The South African Police Service (SAPS) annually releases reported and recorded crime statistics for the preceding financial year i.e. -

It Is Not About The
MyCiTi Bus Service Film Industry Pre-Season Briefing Ronald Kingma Manager IRT Operations Kobus Swanepoel Client Services Transport for Cape Town 11 October 2013 Contents 1. Current System 2. Future Expansion 3. Key Principles 4. Procedure for Filming 5. CCT Fare Policy 6. Application Forms 7. Application Procedure Summary 8. Past film events Current System • Buses : 270 buses • Trunk Routes : Route T01 Wood station – Civic Centre : Route T01a Wood station – V&A Waterfront : Route A01 Airport – V&A Waterfront • Feeder Routes : Route 101 Civic Centre - Gardens : Route 102 Civic Centre – Walmer Estate – Salt River : Route 213 West Beach – Table View - Sunningdale : Route 214 Big Bay – Table View – Parklands : Route 215 Sunningdale- Gie Road - Wood station : Route 216 Sunningdale – Wood Drive – Wood station • Trunk Stations : 17 on Table View, 1 Airport and 4 on Inner City Feeder • Feeder Stops : Inner City: 35 stops : Table View: Over 100 stops • Daily Passengers : 9,000 - 12,000 on weekdays : 2,000 - 5,000 on weekend days • Operation : Approximately 18 hrs per day Future Expansion • 1 November Routes : Route 101 Vredehoek – Gardens - Civic Centre : Route 103 Oranjezicht – Gardens – Civic Centre : Route 104 Sea Point – Waterfront – Civic Centre : Route 105 Sea Point – Fresnaye – Civic Centre : Route 217 Table View - Melkbosstrand : Route 230 Melkbosstrand - Duinefontein • November /Dec : Route 106 Waterfront Silo – Civic Centre – Camps Bay (Clockwise) : Route 107 Waterfront Silo – Civic Centre – Camps Bay (Anti - Clockwise) : Route 251 -
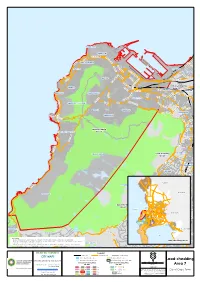
Load-Shedding Area 7
MOUILLE POINT GREEN POINT H N ELEN SUZMA H EL EN IN A SU M Z M A H N C THREE ANCHOR BAY E S A N E E I C B R TIO H A N S E M O L E M N E S SEA POINT R U S Z FORESHORE E M N T A N EL SO N PAARDEN EILAND M PA A A B N R N R D D S T I E E U H E LA N D R B H AN F C EE EIL A K ER T BO-KAAP R T D EN G ZO R G N G A KLERK E E N FW DE R IT R U A B S B TR A N N A D IA T ST S R I AN Load-shedding D D R FRESNAYE A H R EKKER L C Area 15 TR IN A OR G LBERT WOODSTOCK VO SIR LOWRY SALT RIVER O T R A N R LB BANTRY BAY A E TAMBOERSKLOOF E R A E T L V D N I R V R N I U M N CT LT AL A O R G E R A TA T E I E A S H E S ARL K S A R M E LIE DISTRICT SIX N IL F E V V O D I C O T L C N K A MIL PHILIP E O M L KG L SIGNAL HILL / LIONS HEAD P O SO R SAN I A A N M A ND G EL N ON A I ILT N N M TIO W STA O GARDENS VREDEHOEK R B PHILI P KGOSA OBSERVATORY NA F P O H CLIFTON O ORANJEZICHT IL L IP K K SANA R K LO GO E O SE F T W T L O E S L R ER S TL SET MOWBRAY ES D Load-shedding O RH CAMPS BAY / BAKOVEN Area 7 Y A ROSEBANK B L I S N WOO K P LSACK M A C S E D O RH A I R O T C I V RONDEBOSCH TABLE MOUNTAIN Load-shedding Area 5 KLIP PER N IO N S U D N A L RONDEBOSCH W E N D N U O R M G NEWLANDS IL L P M M A A A C R I Y N M L PA A R A P AD TE IS O E R P R I F 14 Swartland RIA O WYNBERG NU T C S I E V D CLAREMONT O H R D WOO BOW Drakenstein E OUDEKRAAL 14 D IN B U R G BISHOPSCOURT H RH T OD E ES N N A N Load-shedding 6 T KENILWORTH Area 11 Table Bay Atlantic 2 13 10 T Ocean R 1 O V 15 A Stellenbosch 7 9 T O 12 L 5 22 A WETTO W W N I 21 L 2S 3 A I A 11 M T E O R S L E N O D Hout Bay 16 4 O V 17 O A H 17 N I R N 17 A D 3 CONSTANTIA M E WYNBERG V R I S C LLANDUDNO T Theewaterskloof T E O 8 L Gordon's R CO L I N L A STA NT Bay I HOUT BAY IA H N ROCKLEY False E M H Bay P A L A I N MAI N IA Please Note: T IN N A G - Every effort has been made to ensure the accuracy of information in this map at the time of puMblication . -

South African Great Escarpment
Sentinel Vision EVT-227 South African Great Escarpment 19 April 2018 Sentinel-1 CSAR IW acquired on 30 August 2017 from 17:17:27 to 17:18:42 UTC Sentinel-2 MSI acquired on 03 September 2017 at 08:19:59 UTC ... Se ntinel-1 CSAR IW acquired on 08 September 2017 from 16:53:05 to 16:53:30 UTC Sentinel-3 SLSTR RBT acquired from 04 January 2018 to 07:59:47 UTC Author(s): Sentinel Vision team, VisioTerra, France - [email protected] 2D Layerstack Keyword(s): Land, mountains, geology, faults, subduction, plateau, orogeny, South Africa Fig. 1 - S2 (03.09.2017) - 11,8,2 colour composite - Zoom on Cape Town region evidencing Table Mountain. 3D view 2D view Table Mountain, Sandstone layers form the ramparts overlying a basement of Precambrian slates and granite - source: Cape Town University Department of Geological Sciences of Cape Town University describes the Geology of the Cape Peninsula: "The late-Precambrian age Malmesbury Group is the oldest rock formation in the area, consisting of alternating layers of dark grey fine-grained greywacke sandstone and slate, seen along the rocky Sea Point and Bloubergstrand shorelines. These sediments were originally deposited on an ancient continental slope by submarine slumping and turbidity currents. The sequence was subsequently metamorphosed by heat and pressure and folded tightly in a NW direction so that the rock layers are now almost vertical. The Peninsula Granite is a huge batholith that was intruded into the Malmesbury Group about 630 million years ago as molten rock (magma) and crystallized deep in the earth, but has since then been exposed by prolonged erosion. -

Groundtruthing Establishing a Citizen Science
GROUNDTRUTHING ESTABLISHING A CITIZEN SCIENCE GROUNDWATER MONITORING NETWORK IN CAPE TOWN !1 © iStock Funder: This project was funded by WWF’s partner, AB InBev Author: GEOSS South Africa (Report number 2019/11-02) GEOSS South Africa is an earth science and groundwater consulting company that specialises in all aspects of groundwater development and management. geoss.co.za Reviewers: Julian Conrad (GEOSS), Dale Barrow (GEOSS), Klaudia Schachtschneider (WWF) Text editing: Marlene Rose Cover photo: © iStock Citation: WWF. 2020. Groundtruthing: Establishing a citizen science groundwater monitoring network in Cape Town. WWF South Africa, Cape Town, South Africa. © Text 2020 WWF South Africa If you would like to share copies of this paper, please do so in this printed or electronic PDF format. Available online at wwf.org.za/report/groundtruthing Published in 2020 by WWF – World Wide Fund for Nature (formerly World Wildlife Fund), Cape Town, South Africa. Any reproduction in full or in part must mention the title and credit the abovementioned publisher as the copyright owner. For more information, contact: Klaudia Schachtschneider Email: [email protected] or Email: [email protected] WWF is one of the world’s largest and most experienced independent conservation organisations with over 6 million supporters and a global network active in more than 100 countries. WWF’s mission is to stop the degradation of the planet’s natural environment and to build a future in which humans live in harmony with nature, by conserving the world’s biological diversity, ensuring that the use of renewable natural resources is sustainable, and promoting the reduction of pollution and wasteful consumption. -

The Great Green Outdoors
MAMRE CITY OF CAPE TOWN WORLD DESIGN CAPITAL CAPE TOWN 2014 ATLANTIS World Design Capital (WDC) is a biannual honour awarded by the International Council for Societies of Industrial Design (ICSID), to one city across the globe, to show its commitment to using design as a social, cultural and economic development tool. THE GREAT Cape Town Green Map is proud to have been included in the WDC 2014 Bid Book, 2014 SILWERSTROOMSTRAND and played host to the International ICSID judges visiting the city. 01 Design-led thinking has the potential to improve life, which is why Cape WORLD DESIGN CAPITAL GREEN OUTDOORS R27 Town’s World Design Capital 2014’s over-arching theme is ‘Live Design. Transform Life.’ Cape Town is defi nitively Green by Design. Our city is one of a few Our particular focus has become ‘Green by Design’ - projects and in the world with a national park and two World Heritage Sites products where environmental, social and cultural impacts inform (Table Mountain National Park and Robben Island) contained within design and aim to transform life. KOEBERG NATURE its boundaries. The Mother City is located in a biodiversity hot Green Map System accepted Cape Town’s RESERVE spot‚ the Cape Floristic Region, and is recognised globally for its new category and icon, created by Design extraordinarily rich and diverse fauna and fl ora. Infestation – the fi rst addition since 2008 to their internationally recognised set of icons. N www.capetowngreenmap.co.za Discover and experience Cape Town’s natural beauty and enjoy its For an overview of Cape Town’s WDC 2014 projects go to www.capetowngreenmap.co.za/ great outdoor lifestyle choices. -

Table Mountain, South Africa
Table Mountain, South Africa Teeming with wildlife and botanical treasures, Table Mountain dominates the skyline of the 'Mother City'. Endlessly pouring tablecloth of clouds amply justifies its riveting presence between the Devil's Peak and Lion's Head sentinels. With its gloomy ravines, rugged cliffs, and indigenous jungles, the mountain creates a magical aura that interweaves with the urban sprawl. As the summit itself offers a visual delight of blue hazed mountains of the hinterland and panoramic views of Robben Island, it is hard not to be enamored of Table Mountain. History Approximately eight hundred million years ago, the mountain was at the sea level. The mountain rose due to the pressure building up in the earth's crust during the Ice Age. About 2000 years ago, it was inhabited by the Khoikhoi people, who were a major tribe when Europeans first arrived in the 16th century. The Khoi and San people named the mountain 'Hoerikwaggo' meaning 'mountain in the sea.' Later, António de Saldanha, who climbed the mountain in 1503, named it 'Taboa do Cabo' meaning “Table of the Cape.” Table Mountain Activities Ride Table Mountain Cableway – The Table Mountain Aerial Cableway was opened in October 4, 1929. It's a much safer, faster, and convenient 1200-meter ride that carries visitors from the Lower Cable Station on Kloofnek Road to the top of Table Mountain. Experience the sunny days and balmy evenings atop the mountain by taking a cable car to the summit, and be amazed by the jaw-dropping views of Cape Town, Robben Island, and the Cape Peninsula. -

What Lies Beneath Table Mountain Or All Models Are Wrong, but Some Are Useful
WHAT LIES BENEATH TABLE MOUNTAIN OR ALL MODELS ARE WRONG, BUT SOME ARE USEFUL Prof Alexander Kisters August 2016 WHAT LIES BENEATH TABLE MOUNTAIN OR ALL MODELS ARE WRONG, BUT SOME ARE USEFUL Inaugural lecture delivered on 2 August 2016 Prof Alexander Kisters Department of Earth Sciences Faculty of Science Stellenbosch University Editor: SU Language Centre Printing: SUN MeDIA ISBN: 978-0-7972-1614-3 Copyright © 2016 Alexander Kisters BIOGRAPHY rofessor Alexander (Alex) Kisters completed his Pgeology undergraduate studies and MSc at the Aachen University of Technology (RWTH) in Germany and obtained a PhD in geology from the University of the Witwatersrand, Johannesburg. Subsequently, he had worked in the mineral exploration industry in South Africa and had held various postdoctoral and lecturer positions at the universities of Aachen, Cologne and the Witwatersrand before joining Stellenbosch University in 1999. Currently, he is appointed at the Department of Earth Sciences, University of Stellenbosch, as a professor in structural geology and tectonics where he teaches under- and postgraduate courses. He has supervised and cosupervised over 50 honours students, 15 MSc studies and 7 PhD studies. Alex has held a National Research Foundation rating for the past 15 years and a B-rating for the last five years. His research focuses on a range of topics related to crustal deformation aimed at integrating field data with regional tectonic models and more generic processes of melt transport and hydrothermal fluid flow in the Earth’s crust. 1 ABSTRACT he geological evolution of the Western Cape remains elusive after over 100 years of research. Based on regional Tfieldwork and correlations, this contribution aims to develop a tectonic model for the deposition and deformation of rocks of the Malmesbury Group, the rocks that underlie much of the Western Cape. -

Cederberg-IDP May 2020 – Review 2020-2021
THIRD REVIEW: 2020/2021 MAY 2020 SECTIONS REVISED THIRD REVISION TO THE FOURTH GENERATION IDP ................... 0 3.8. INTERGOVERNMENTAL RELATIONS ................................. 67 FOREWORD BY THE EXECUTIVE MAYOR.................................. 2 3.9. INFORMATION AND COMMUNICATION TECHNOLOGY (ICT) ...... 68 ACKNOWLEDGEMENT FROM THE MUNICIPAL MANAGER AND IMPORTANT MESSAGE ABOUT COVID-19 ................................. 4 CHAPTER 4: STRATEGIC OBJECTIVES AND PROJECT ALIGNMENT .. 71 EXECUTIVE SUMMARY ....................................................... 5 4.1 IMPROVE AND SUSTAIN BASIC SERVICE DELIVERY AND CHAPTER I: STATEMENT OF INTENT ...................................... 9 INFRASTRUCTURE .................................................... 73 1.1. INTRODUCTION ......................................................... 9 A. Water B. Electricity 1.2. THE FOURTH (4TH) GENERATION IDP .............................. 10 C. Sanitation D. Refuse removal / waste management 1.3. THE IDP AND AREA PLANS ........................................... 11 E. Roads F. Comprehensive Integrated Municipal Infrastructure Plan 1.4. POLICY AND LEGISLATIVE CONTEXT ................................ 11 G. Stormwater H. Integrated Infrastructure Asset Management Plan 1.5. STRATEGIC FRAMEWORK OF THE IDP .............................. 13 I. Municipal Infrastructure Growth Plan 1.6. VISION, MISSION, VALUES ............................................ 14 4.2 FINANCIAL VIABILITY AND ECONOMICALLY SUSTAINABILITY .... 87 1.7. STRATEGIC OBJECTIVES ............................................